The Art of War
The concept of the bowel as noxious reservoir first appeared in the early years of the twentieth century, when a Scottish surgeon William Arbuthnot-Lane began performing total colectomies in patients with chronic constipation, to prevent “autointoxication” from toxic bowel contents (1). This practice was abandoned (along with the surgeon), but the concept of autointoxication has been revived, and the bowel is now recognized as a leading source of morbidity and mortality in critically ill patients.
This chapter describes abdominal infections that occur in the ICU, including infections of the biliary tree (acalculous cholecystitis), bowel (Clostridium diffcile enterocolitis), and peritoneal cavity (postoperative infections) (2,3).
ACALCULOUS CHOLECYSTITIS
Acalculous cholecystitis accounts for only 5–15% of cases of acute cholecystitis (4), but it is more common in critically ill patients, and has a mortality rate (about 45%) that rivals that of septic shock (4,5).
Pathogenesis
Common conditions associated with acalculous cholecystitis include the postoperative period (especially following cardiopulmonary bypass surgery), trauma, circulatory shock, and multiorgan failure (4,5). Prolonged bowel rest (i.e., during total parenteral nutrition) predisposes to acalculous cholecystitis by promoting cholestasis, but as much as 4 weeks of bowel rest may be required before acalculous cholecystitis is a risk (6), which is longer than the ICU stay of most patients.
Possible underlying mechanisms for acalculous cholecystitis include hypoperfusion, gallbladder distension from diminished contractions, and a change in the composition of bile. The latter mechanism may have an important role, because biliary “sludge” (i.e., echogenic matter in the gallbladder associated with acalculous cholecystitis) contains small crystals called “microliths” that can produce cholecystitis (5).
Clinical Features
Most cases of acalculous cholecystitis are not discovered until complications arise (e.g., gangrenous cholecystitis or gallbladder perforation), so the clinical manifestations reported for acalculous cholecystitis are often those of advanced, complicated cholecystitis. The diagnosis of acalculous cholecystitis is often delayed because pain and tenderness in the right upper quadrant can be absent in one-third of patients with acalculous cholecystitis (2). Fever (100%), elevated bilirubin (90%), hypotension (90%), and multiorgan failure (65–80%) are common but nonspecific findings (4,5). Blood cultures are positive in 90% of cases (2) and Gram-negative aerobic bacilli are isolated in almost all cases.
Diagnosis
Ultrasound is the favored diagnostic test for acalculous cholecystitis because it can be performed at the bedside. Gallbladder distension and sludge are suggestive findings, but are nonspecific. The ultrasound image in Figure 40.1 shows more specific findings; i.e., marked thickness of the gallbladder wall and sloughed mucosa in the lumen of the gallbladder. The diagnostic yield from ultrasound varies widely in different reports (4,8), and may be operator-specific. If ultrasound is not helpful, the next step is a hepatobiliary scan, which is the “gold standard” method for the diagnosis of acute cholecystitis (but requires a functional liver to move the tracer into the bile ducts).
Management
Prompt intervention is mandatory. Cholecystectomy is the procedure of choice, but for patients who are too unstable for surgery, percutaneous drainage of the gallbladder is a suitable alternative. Empiric antibiotic therapy should be started as soon as the diagnosis is confirmed. The recommended antibiotics are piperacillin-tazobactam, or a carbapenem (imipenem or meropenem) (2).
COLONIZATION OF THE GI TRACT
The microbial landscape in the GI tract is altered in critically ill patients, and the infections that can appear as a result of this change are described in this section.
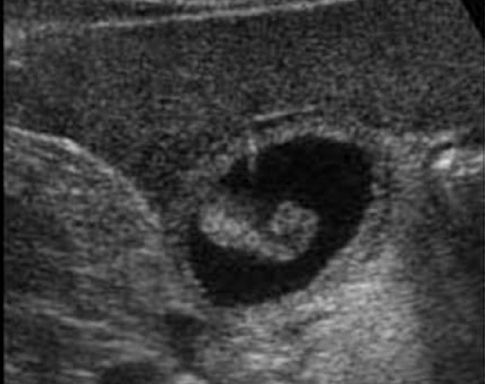
FIGURE 40.1 Transverse sonogram of the gallbladder showing marked thickening of the gallbladder wall and an echogenic mass projecting into the lumen of the gallbladder. This mass represents sloughed mucosa, and is characteristic of gangrenous cholecystitis.
Gastric Colonization
Because bacteria do not thrive in an acid environment (see Figure 5.3 on page 81), gastric acidity maintains a sterile environment in the stomach. Loss of gastric acidity (from acid-suppressing drugs used to prevent stress ulcer bleeding) promotes colonization of the stomach, and the following observations indicate that gastric colonization increases the risk of nosocomial infections.
1. The use of acid-suppressing drugs for stress ulcer prophylaxis is associated with an increased incidence of nosocomial pneumonia (9).
2. Translocation of organisms has been documented in 15% of cases of gastric colonization, and about half of the cases of translocation resulted in a nosocomial infection (10).
3. The organisms isolated most often from the stomach are the same as the organisms isolated most often in nosocomial infections (11). This is shown in Figure 40.2.
Corrective Measures
There are two measures that could reduce colonization of the stomach: (a) avoiding the use of gastric acid-suppressing drugs for prophylaxis of stress ulcer bleeding, and (b) selective digestive decontamination with nonabsorbable antibiotics. Both of these measures are presented in Chapter 5.
Clostridium difficile
Clostridium difficile is a spore-forming, Gram-positive anaerobic bacillus that does not inhabit the bowel in healthy subjects, but is able to colonize and proliferate in the bowel when the normal microflora has been altered by antibiotic therapy (12). The typical host for C. difficile colonization is an elderly or debilitated patient or nursing home resident who has received antibiotics at some time in the last 2 weeks. Colonization is uncommon in healthy subjects who live in the community (although this may change).
Pathogenesis
C. difficile is transmitted from patient to patient via the fecal-oral route. There is a dormant (spore) form that can survive on environmental surfaces for months, but patient-to-patient transmission usually occurs via the hands of hospital personnel (13). As a result, strict adherence to the use of disposable gloves can significantly reduce transmission (14).
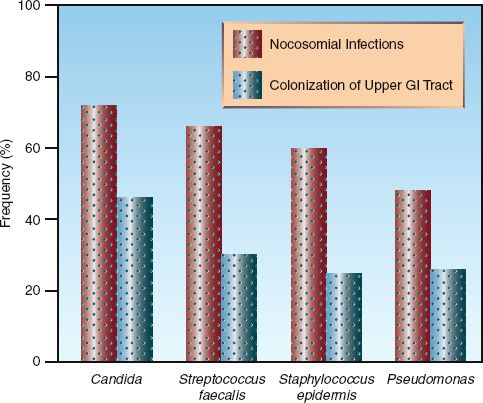
FIGURE 40.2 Correlation between the most frequent isolates in the stomach and the most frequent isolates in nosocomial infections in critically ill patients. Data from Reference 4.
C. difficile is not an invasive organism, but releases cytotoxins that damage the bowel mucosa. This leads to inflammatory infiltration of the bowel wall and symptomatic disease. Severe inflammation is accompanied by raised, plaque-like lesions on the mucosal surface known as “pseudomembranes.” The presence of these lesions (pseudomembranous colitis) is evidence of severe disease.
GASTRIC ACID SUPPRESSION: There are several reports showing that use of acid-suppressing drugs, particularly proton pump inhibitors, is associated with an increased risk of C. difficile infection (15–17). The risk of other enteric infections (e.g., salmonellosis) is also increased by loss of gastric acidity (18), and this effect is further evidence of the role of gastric acid as an antimicrobial defense mechanism.
The protective effect of gastric acid on C. difficile infections has important implications because of the escalating and excessive use of gastric acid-suppressing drugs, particularly proton pump inhibitors, in hospitalized patients. In fact, there has been a marked increase in the frequency and severity of C. difficile infections in recent years (19), and this coincides with the marked increase in the use of proton pump inhibitors for prophylaxis of stress ulcer bleeding. Therefore, it is possible that the recent surge in C. difficile infections is a reflection of the escalating (and unnecessary) use of proton pump inhibitors in hospitalized patients (20).
Clinical Features
The principal manifestation of Clostridium difficile infection (CDI) is a watery diarrhea, which can occur alone (in mild cases) or in combination with fever and leukocytosis (in more severe cases), and can progress to circulatory shock and multiorgan failure. The feared (but uncommon) complication of CDI is toxic megacolon, which presents with abdominal distension, circulatory shock, and an x-ray that looks like Figure 40.3.
Diagnostic Evaluation
The diagnosis of CDI requires evidence of C. difficile cytotoxins in stool. Stool cultures for C. difficile are unreliable because they do not distinguish toxigenic from nontoxigenic strains of the organism. Most laboratories use an ELISA (Enzyme-Linked Immunosorbent Assay) method to detect cytotoxins. The sensitivity of this test is about 85% for one stool specimen and up to 95% for 2 stool specimens (12,21,22). Therefore, the cytotoxin assay will miss 15% of diagnoses if one stool specimen is tested, and only misses 5% of diagnoses if two stool specimens are tested. The specificity of this test is up to 98% (21), so false-positive results are uncommon.
COLONOSCOPY: Direct visualization of the bowel mucosa is reserved for the occasional case where there is a high clinical suspicion of C. difficile infection that is not confirmed by cytotoxin assays. The presence of pseudomembranes confirms the diagnosis of C. difficile infection. Colonoscopy is preferred to proctosigmoidoscopy for optimal results.
Antibiotic Treatment
The first step in treating CDI is to discontinue any predisposing drugs (antibiotics and proton pump inhibitors), if possible. Antiperistaltic agents should also be discontinued because reduced peristalsis can prolong the exposure to C. difficile cytotoxins (12). The recommended antibiotic regimens for CDI are shown in Table 40.1. Treatment is organized by the clinical condition (mild, severe, or relapsed CDI), and by the ability to give PO (or nasogastric) medications.
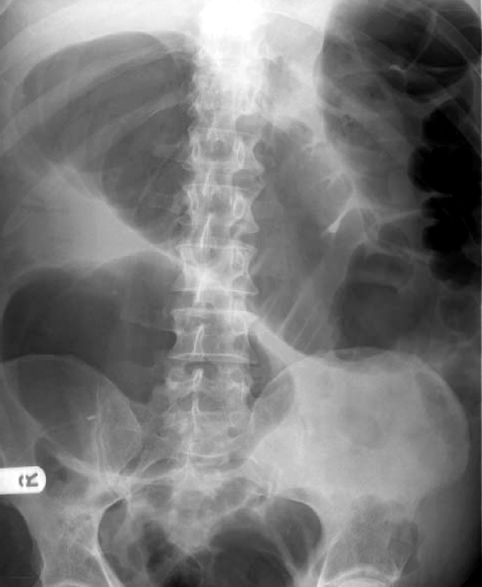
FIGURE 40.3 Radiographic appearance of toxic megacolon in a patient with C. difficile enterocolitis.
Table 40.1 Antibiotic Therapy for Clostridium difficile Infections (CDI)
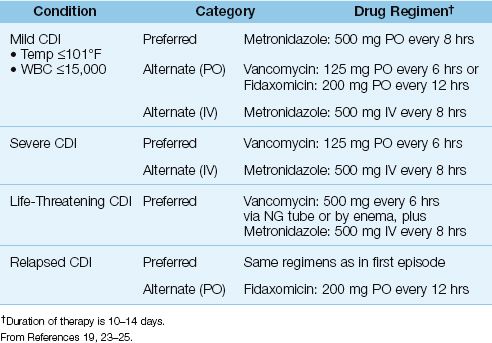
MILD CDI: Mild CDI is defined as cytotoxin-positive diarrhea with a body temperature no higher than 101°F and a blood leukocyte count no higher than 15,000/mm3 (19,23,24). The preferred treatment is oral metronidazole (500 mg every 8 hrs) for 10–14 days (23,24). Oral vancomycin (125 mg every 6 hrs) is equally effective, but is used as a second-line agent to limit proliferation of vancomycin-resistant enterococci. Fidaxomicin is a re-cently-introduced antibiotic that is equivalent to vancomycin for treating an acute episode of CDI, and has 50% fewer relapses (25).
SEVERE CDI: Severe CDI is defined as cytotoxin-positive diarrhea with any of the following: (a) a body temperature above 101°F and a blood leukocyte count above 15,000/mm3, (b) the presence of pseudomembranes, or (c) a complication of CDI (e.g., toxic megacolon, renal failure, septic shock). The treatment of choice for severe CDI is oral vancomycin (125 mg every 6 hrs), which is more effective than oral metronidazole (24). For patients who are critically ill from CDI, the recommended treatment is vancomycin in a dose 500 mg (via NG tube or enema) plus IV metronidazole (500 mg every 8 hrs) (23).
RESPONSE: In most cases, the fever resolves in 24–48 hrs, and the diarrhea resolves in 4–5 days (12). Treatment is continued for 10–14 days. Per-sistence of symptomatic disease occurs with complications like toxic megacolon, peritonitis, or progressive sepsis and multiorgan failure, which often require surgical intervention (26). The procedure of choice is subtotal colectomy.
RELAPSES: Relapses (usually within 3 weeks) are reported in 25% of cases treated with metronidazole or vancomycin (12,24), and 13% of cases treated with fidaxomicin (24). Repeat therapy using the same antibiotic is successful in about 75% of relapses, and another relapse is expected in about 25% of cases (27). Fewer relapses are reported with fidaxomicin (25), which may become the preferred treatment for recurrent CDI. About 5% of patients experience more than 6 relapses (12).
Microbial Therapy
Microbial therapy is used for recurrent CDI, and is an attempt to restore the normal microflora of the bowel to antagonize or prevent colonization with C. difficile.
PROBIOTICS: Probiotics are non-pathogenic organisms (Saccharomyces boulardii or Lactobacillus species) that bind to epithelial cells and prevent the attachment of C. difficile. Probiotic therapy with S. boulardii (1 g/day, started with antimicrobial therapy and continued for 4 weeks), but not Lactobacillus, can reduce the incidence of recurrent CDI (23). Therefore, probiotic therapy with S. boulardii can be used as adjunctive therapy for CDI to prevent recurrences (23).
FECAL TRANSPLANTATION: Instillation of liquid preparations of stool from healthy donors (via nasogastric tube or enemas) has proven successful in treating recurrent CDI in 70–100% of cases (23,28). (For a description of the fecal transplantation process, including donor screening, see Reference 28.)
POSTOPERATIVE INFECTIONS
Postoperative abdominal infections are located in the peritoneal cavity, and are the result of peritoneal seeding during the procedure, or leakage of bowel contents from an anastomotic site or undetected injury to the bowel wall. These infections can present as diffuse peritonitis or an abdominal abscess.
Peritonitis
Generalized peritonitis is not a common presentation for postoperative infection, and is usually the result of an undetected tear in the bowel wall during the procedure.
Clinical Features
Small tears often present with non-specific abdominal pain initially, and the first sign of a tear may be the presence of air under the diaphragm, as shown in Figure 40.4. As little as 1 mL of gas can be detected under the right hemidiaphragm in the upright position (29). The presence of air under the diaphragm may not, however, be a useful finding soon after a laparoscopic procedure, because the instillation of CO2 during laparo-scopy can result in residual air under the diaphragm for days.
A persistent leak through a tear in the bowel wall will eventually produce signs of peritoneal irritation (i.e., involuntary guarding and rebound tenderness) and a systemic inflammatory response (fever, leukocytosis, etc.). Progression to circulatory shock (e.g., hypotension, change in mental status) can be rapid.
Management
Signs of diffuse peritonitis merit immediate surgical exploration. The initial management should include the following measures.
FLUIDS: Peritonitis is often accompanied by considerable fluid loss into the peritoneal cavity, so signs of circulatory compromise (i.e., a decrease in urine output or blood pressure) should prompt aggressive volume resuscitation. Avoiding vasopressor therapy is advised, whenever possible, because vasopressors promote splanchnic vasoconstriction and can aggravate an underlying ischemic condition in the bowel.
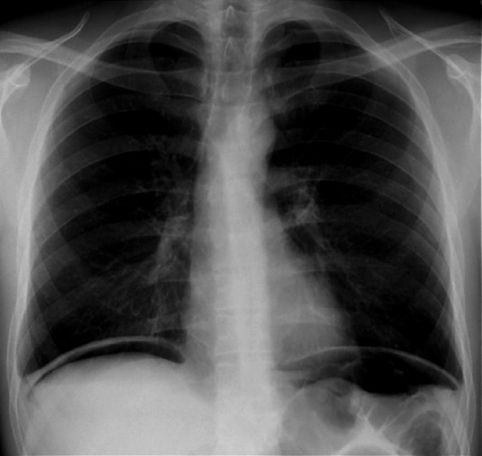
FIGURE 40.4 Abdominal x-ray in the upright position showing free air under both hemidiaphragms. In the absence of a recent laparoscopy, this finding is evidence of a perforated hollow viscus.
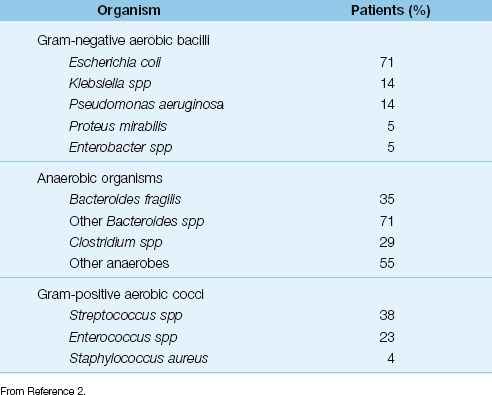
ANTIBIOTICS: Antibiotic therapy should be started as soon as possible using antibiotics that are active against the frequent isolates as presented in Table 40.2. Single-agent antibiotic coverage using piperacillin-tazobactam, or a carbapenem (imipenem-cilastatin, meropenem, or doripenem) is recommended (2). In patients who may be colonized by Candida species (e.g., patients who have received antibiotics recently), additional empiric coverage with an antifungal agent (e.g., fluconazole) seems wise.
Table 40.2 Organisms Isolated in 1,237 Patients With Complicated Abdominal Infections
Abdominal Abscess
Abdominal abscesses typically serve as an occult source of sepsis, and are difficult to detect with routine clinical evaluations.
Clinical Features
Fever is almost always present (30), but localized abdominal tenderness can be absent in 60% of cases, and a palpable abdominal mass is evident in less than 10% of cases (30,31). Abdominal x-rays can show extraluminal air, but this occurs in less than 15% of cases (31).
Computed Tomography
Computed tomography (CT) of the abdomen is the most reliable diagnostic method of detection for abdominal abscesses, with a sensitivity and specificity of 90% or higher (31). However, CT imaging in the early postoperative period can be misleading because collections of blood or irrigant solutions in the peritoneal cavity can be misread as an abscess. CT scans are most reliable when performed after the first postoperative week (when peritoneal fluid collections have resorbed) (31). The CT appearance of an abdominal abscess is shown in Figure 40.5.
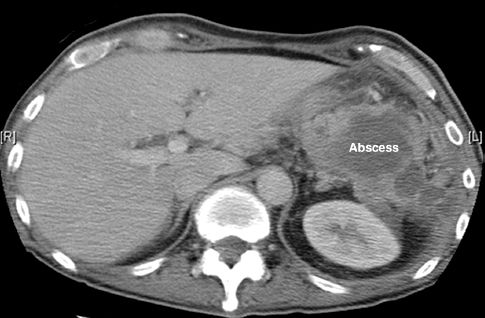
FIGURE 40.5 Abdominal CT scan showing a multiloculated abscess in the left upper quadrant in a post-splenectomy patient.
Management
Immediate drainage is advised for postoperative abdominal abscesses. Precise localization with CT imaging allows many abscesses to be drained percutaneously with CT-guided drainage catheters (30). Empiric antibiotic therapy should be started while awaiting the results of abscess fluid cultures. The empiric antibiotic regimen is the same as described for peritonitis.
A FINAL WORD
Spotlight on Gastric Acid
As mentioned often in this book, the role of gastric acid as an antimicrobial defense mechanism has not received the attention it deserves. This is particularly true regarding the observation that suppression of gastric acidity with proton pump inhibitors promotes the transmission of Clostridium difficile infections. In fact, the recent surge in the incidence and severity of C. difficile infections may be a reflection of the escalating (and unnecessary) use of proton pump inhibitors for prophylaxis of stress ulcer bleeding (20).
The following observation should help you to avoid proton pump inhibitors for stress ulcer prophylaxis.
1. Proton pump inhibitors are not more effective than H2 blockers (e.g., ranitidine) for preventing stress ulcer bleeding (33).
And the following observation should help you to avoid any type of gastric acid-suppressing drug for stress ulcer prophylaxis.
2. When patients are receiving enteral tube feedings for full nutritional support, there is no added benefit from gastric acid-suppressing drugs for the prevention of stress ulcer bleeding (34).
REFERENCES
Introduction
1. Arbuthnot-Lane W. Remarks on the operative treatment of chronic constipation. Reprinted in Dis Colon & Rectum 1985; 28:750–757.
Reviews
2. Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Disease Society of America. Clin Infect Dis 2010; 50:133–164.
3. Sarteli M, Viale P, Catena F, et al. 2013 WSES guidelines for the management of intra-abdominal infections. World J Emerg Surg 2013; 8:3.
Acalculous Cholecystitis
4. McChesney JA, Northrup PG, Bickston SJ. Acute acalculous cholecystitis associated with systemic sepsis and visceral arterial hypoperfusion. A case series and review of pathophysiology. Dig Dis Sci 2003; 48:1960–1967.
5. Laurila J, Syrjälä H, Laurila PA, et al. Acute acalculous cholecystitis in critically ill patients. Acta Anesthesiol Scand 2004; 48:986–991.
6. Messing B, Bories C, Kuntslinger C. Does parenteral nutrition induce gallbladder sludge formation and lithiasis? Gastroenterology 2983; 84:1012–1019.
7. Jüngst C, Killack-Ublick GA, Jüngst D. Gallstone disease: microlithiasis and sludge. Best Prect Res Clin Gastroenterol 2006; 20:1053–1062.
8. Puc MM, Tran HS, Wry PW, Ross SE. Ultrasound is not a useful screening tool for acalculous cholecystitis in critically ill trauma patients. Am Surg 2002; 68:65–69.
Gastric Colonization
9. Huang J, Cao Y, Liao C, et al. Effect of histamine-2-receptor antagonists versus sucralfate on stress ulcer prophylaxis in mechanically ventilated patients: A meta-analysis of 10 randomized controlled trials. Crit Care 2010; 14:R194–R204.
10. MacFie J, Reddy BS, Gatt M, et al. Bacterial translocation studied in 927 patients over 13 years. Br J Surg 2006; 93:87–93.
11. Marshall JC, Christou NV, Meakins JL. The gastrointestinal tract: the “un-drained abscess” of multiple organ failure. Ann Surg 1993; 218:111–119.
Clostridium difficile Infections
12. Bartlett JG. Antibiotic-associated diarrhea. N Engl J Med 2002; 346:334–339.
13. Samore MH, Venkataraman L, DeGirolami, et al. Clinical and molecular epidemiology of sporadic and clustered cases of nosocomial Clostridium difficile diarrhea. Am J Med 1996; 100:32–40.
14. Johnson S, Gerding DN, Olson MM, et al. Prospective, controlled study of vinyl glove use to interrupt Clostridium difficile nosocomial transmission. Am J Med 1990; 88:137–140.
15. Dial S, Alrasadi K, Manoukian C, et al. Risk of Clostridium-difficile diarrhea among hospitalized patients prescribed proton pump inhibitors: cohort and case-control studies. Canad Med Assoc J 2004; 171:33–38.
16. Dial S, Delaney JA, Barkun AN, Suissa S. Use of gastric acid-suppressing agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA 2005; 294:2989–2995.
17. Aseri M, Schroeder T, Kramer J, Kackula R. Gastric acid suppression by proton pump inhibitors as a risk factor for Clostridium difficile-associated diarrhea in hospitalized patients. Am J Gastroenterol 2008; 103:2308–2313.
18. Cook GC. Infective gastroenteritis and its relationship to reduced gastric acidity. Scand J Gastroenterol 1985; 20(Suppl 111):17–21.
19. Kelly CP, LaMont JT. Clostridium difficile – more difficult than ever. N Engl J Med 2008; 359:1932–1940.
20. Cunningham R, Dial S. Is over-use of proton pump inhibitors fuelling the current epidemic of Clostridium-difficile-associated diarrhea? J Hosp Infect 2008; 70:1–6.
21. Mylonakis E, Ryan ET, Calderwood SB. Clostridium difficile-associated diarrhea. Arch Intern Med 2001; 161:525–533.
22. Yassin SF, Young-Fadok TM, Zein NN, Pardi DS. Clostridium difficile– associated diarrhea and colitis. Mayo Clin Proc 2001; 76:725–730.
23. van Nispen tot Pannerden CMF, Verbon A, Kuipers E. Recurrent Clostridium difficile infection. What are the treatment options. Drugs 2011; 71:853–868.
24. Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis 2007; 45:302–307.
25. Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 2011; 364:422–431.
26. Lipsett PA, Samantaray DK, Tam ML, et al. Pseudomembranous colitis: a surgical disease? Surgery 1994; 116:491–496.
27. Aslam S, Hamill RJ, Musher DM. Treatment of Clostridium difficile-associated disease: old therapies and new strategies. Lancet Infect Dis 2005; 5:549–557.
28. Aas J, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via nasogastric tube. Clin Infect Dis 2003; 36:580–585.
Complicated Abdominal Infections
29. Miller RE, Nelson SW. The roentgenologic demonstration of tiny amounts of free intraperitoneal gas: experimental and clinical studies. AJR Am J Roentgenol 1971; 112:574–585.
30. Khurrum Baig M, Hua Zao R, Batista O, et al. Percutaneous postoperative intra-abdominal abscess drainage after elective colorectal surgery. Tech Coloproctol 2002; 6:159–164.
31. Fry DE. Noninvasive imaging tests in the diagnosis and treatment of intra-abdominal abscesses in the postoperative patient. Surg Clin North Am 1994; 74:693–709.
A Final Word
32. Lin P-C, Chang C-H, Hsu P-I, et al. The efficacy and safety of proton pump inhibitors vs. histamine-2 receptor antagonists for stress ulcer bleeding prophylaxis among critical care patients: A meta-analysis. Crit Care Med 2010; 38:1197–1205.
33. Marik PE, Vasu T, Hirani A, Pachinburavan M. Stress ulcer prophylaxis in the new millennium: a systematic review and meta-analysis. Crit Care Med 2010; 38:2222–2228.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








