DEFINITION AND HISTORY
The first prospective safety study of intrathecal lidocaine was performed by Phillips et al.4 and published in 1968. This study evaluated 10,440 patients (93% obstetric) undergoing spinal anesthesia with lidocaine. The authors concluded that lidocaine was safe for spinal anesthesia. Analyses of these data reveal that during the study period 284 patients complained of back pain. Of these patients, 91 refused subsequent spinal anesthesia because of postspinal back pain. Subsequently, millions of patients underwent spinal anesthesia with 5% hyperbaric lidocaine, with only rare published reports of complications. Scrutiny of lidocaine began in 1991, with case reports documenting CES after continuous spinal anesthesia.1,2 Of the initial case reports of CES following continuous spinal anesthesia, all but one involved the use of lidocaine. It was postulated that the mechanics of spinal microcatheters (which allowed the pooling of local anesthetic at the lumbosacral roots), in combination with an extremely large dose of the local anesthetic, were the cause of CES in these patients. Subsequently, at the direction of the United States (US) Food and Drug Administration (FDA), spinal microcatheters were withdrawn from the US market.
Concern over the use of single-dose 5% hyperbaric lidocaine for spinal anesthesia began in 1993 when Schneider et al.3 published a report of four patients undergoing spinal anesthesia in the lithotomy position who experienced postoperative aching and pain in the buttocks and lower extremities. Initial reports used the term transient radicular irritation to describe this syndrome. Eventually, the terminology was changed to TNS to better reflect the symptomatology and lack of definitive etiology. All of Schneider’s patients recovered completely. Nonetheless, subsequent case reports and editorials questioned the continued use of 5% hyperbaric lidocaine and suggested that a fresh appraisal of its safety by the appropriate regulatory agencies might be in order.5,6
The term TNS is used to describe both unilateral and bilateral pain occurring within 24 hours after spinal anesthesia that involves the buttocks and may radiate to the lower extremities. The lower back may or may not be included. The term TNS is itself controversial because it implies a neurologic etiology, which has yet to be proven (Box 13-1).
BOX 13-1 Diagnostic Components of TNS
 Symptoms begin within 24 hours after the resolution of spinal anesthesia.
Symptoms begin within 24 hours after the resolution of spinal anesthesia.
 Most patients experience aching and/or pain of the unilateral or bilateral buttocks. Fewer patients will experience dysesthesia radiating into the anterior or posterior thighs.
Most patients experience aching and/or pain of the unilateral or bilateral buttocks. Fewer patients will experience dysesthesia radiating into the anterior or posterior thighs.
 Back pain may not be present in all patients.
Back pain may not be present in all patients.
 Symptoms resolve in 6 hours to 4 days.
Symptoms resolve in 6 hours to 4 days.
 There are no neurologic findings upon physical exam or imaging.
There are no neurologic findings upon physical exam or imaging.
 SCOPE
SCOPE
After Schneider’s initial case report, multiple case reports and a few laboratory and clinical studies evaluated the components of TNS after spinal anesthesia. Prospective randomized controlled studies7–25 have shown a remarkable variability in the incidence of TNS among patients undergoing spinal anesthesia with lidocaine (Fig. 13-1). Clearly, the incidence of TNS is the highest following lidocaine spinal anesthesia versus other local anesthetics. The association of TNS with lidocaine spinal anesthesia has also been supported by two separate meta-analyses.26,27 Randomized studies, as well as an epidemiologic study by Freedman et al.,28 have shown that the incidence of TNS varies with the type of surgery performed (Table 13-1). For example, patients undergoing surgery in the lithotomy position have an incidence of TNS of approximately 30% to 36%,7,9,12 patients undergoing arthroscopic knee surgery an incidence of 18% to 22%,8,10,15,16 and patients undergoing surgery in the supine position an incidence of 4% to 8%.18,19 This observation makes it easier to explain the variation reported in studies evaluating the incidence of TNS.
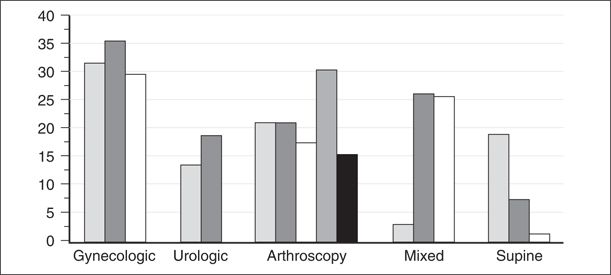
FIGURE 13-1. Incidence of TNS as a function of surgical position. All patients received spinal lidocaine. Most gynecologic and urologic patients were in lithotomy position. All arthroscopy patients were knee arthroscopy. Mixed patients refer to studies where surgical position varied. Vertical bars represent individual studies, which are referenced to the following citations: gynecologic,7,9,12 urologic,13,24 knee arthroscopy,8,10,15,16,25 mixed surgery,11,14,17 and supine.8,19,20
TABLE 13.1 Randomized Controlled Studies of TNS
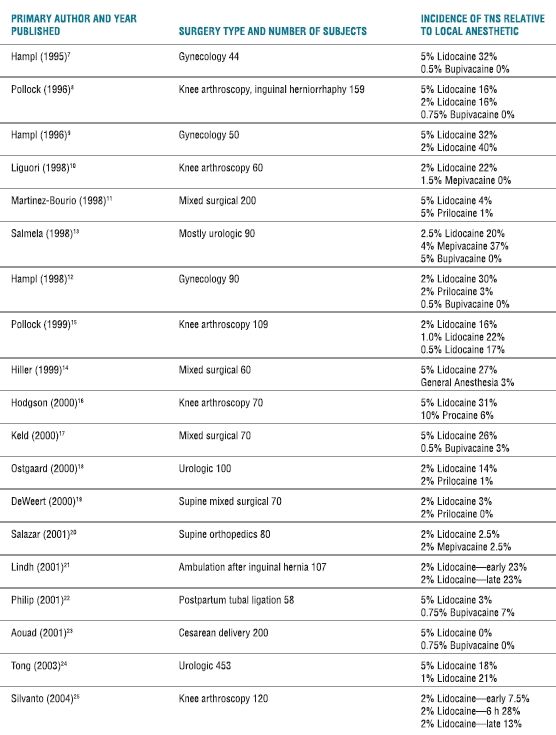
Because the symptoms of TNS are transitory and result in no abnormalities on physical exam or neurologic testing, many physicians have questioned the clinical importance of the syndrome. One study attempted to quantify the degree of functional impairment found in patients experiencing TNS24 by randomizing 453 patients to undergo either 1% or 5% hyperbaric 80-mg lidocaine spinal anesthesia for urologic surgery. The patients were assessed for the incidence of TNS and functional impairment. The incidence of TNS was 21% with 1% lidocaine and 18% with 5% lidocaine, and the patients experiencing TNS reported significant impairment of daily functional activities such as walking, sitting, and sleeping.
 PATHOPHYSIOLOGY
PATHOPHYSIOLOGY
Possible causes of TNS include specific local anesthetic toxicity,5,6 needle trauma, neural ischemia secondary to sciatic stretching,3 patient positioning, pooling of local anesthetics secondary to small-gauge pencil-point needles,29 muscle spasm, myofascial trigger points,30 early mobilization, and/or irritation of the dorsal root ganglion.31 Because few patients receiving intrathecal bupivacaine report TNS, it appears that TNS is not the result of having a subarachnoid block per se. Hence, epiphenomena of subarachnoid block (spinal needle placement, bed-to-bed transfer, or surgery) are not the sole causative factors of TNS.32
Several authors have assumed that TNS is a symptom of direct neurotoxicity. Local anesthetics clearly exert significant neurotoxicity in laboratory models, and indeed lidocaine, tetracaine, and prilocaine seem to be more neurotoxic in animal models than are bupivacaine and chloroprocaine.33 Concentrations of lidocaine within its clinically useful range (1%–5%) have been shown to inhibit nerve conduction in isolated frog sciatic nerve models.34,35 However, one argument against local anesthetic toxicity as the etiology of TNS is that factors reported to increase the incidence of TNS are not the same factors that increase the incidence of CES, which is known to result from local anesthetic toxicity. For example, the incidence of CES is increased by higher doses and concentrations of local anesthetics and by the addition of vasoconstrictors, but none of these factors appear to increase the incidence of TNS. One study attempted to determine if TNS was the result of direct neurotoxicity of lidocaine by evaluating volunteers with electromyography, nerve conduction studies, and somatosensory-evoked potentials before and during episodes of TNS. Volunteers in this small study had no abnormalities in electrophysiologic tests even in areas susceptible to the effects of local anesthetic toxicity such as the posterior nerve roots.36
The etiology of TNS has been the subject of ongoing laboratory and clinical research. It does appear that TNS is associated predominately with the use of lidocaine spinal anesthesia, that decreasing the concentration from 5% to 0.5% does not decrease the incidence of TNS,15,24 and that hyperosmolarity,7 hyperbaricity, or the addition of glucose are not contributing factors. Why surgical position contributes to the development of TNS remains unclear, but potential etiologies include musculoskeletal strain or sciatic nerve stretching.
 RISK FACTORS
RISK FACTORS
Clinical studies have attempted to determine which patients may be at risk for the development of TNS. Lidocaine spinal anesthesia and the lithotomy position24 are important contributing factors. In the epidemiologic study by Freedman et al.,28 outpatient status was shown to be a significant risk factor for the development of TNS, but subsequent randomized controlled studies21,25 have not confirmed early ambulation as an additional risk factor. One of these studies21 randomized inguinal hernia patients to early (immediate) or late (12 hours) ambulation following 100-mg hyperbaric 2% lidocaine spinal anesthesia and found no difference in the incidence of TNS (23%) between the groups. Arthroscopic knee surgery and obesity may also affect the incidence of TNS (Box 13-2).
BOX 13-2 Primary Risk Factors for TNS
 Lidocaine > other local anesthetics >> bupivacaine.
Lidocaine > other local anesthetics >> bupivacaine.
 Patient position: lithotomy or knee arthroscopy.
Patient position: lithotomy or knee arthroscopy.
 Ambulation and obesity: conflicting data.
Ambulation and obesity: conflicting data.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


