Fig. 24.1
The daVinci® surgical cart (Intuitive Surgical, Mountain View, CA) during a procedure
Although the daVinci surgical robot was originally marketed for cardiac surgical applications, it has found widespread use in urologic surgery. In addition to urology, the daVinci Robot is used in cardiac, thoracic, general surgical, and gynecologic surgical applications.
The enhanced dexterity of the daVinci Robot has enabled the application of video-assisted thoracic surgery to complex procedures such as (1) totally endoscopic lobectomy for lung cancer; (2) endoscopic esophagectomy for esophageal cancer; (3) endoscopic resection of mediastinal masses; (4) endoscopic thymectomy for myasthenia gravis; and (5) endoscopic transthoracic esophageal myotomy for achalasia.
Totally Endoscopic Robotic Pulmonary Lobectomy for Lung Cancer
Lobectomy remains the standard of care for the treatment of early-stage non-small-cell lung cancer. Until recently, thoracotomy has been the standard approach to lobectomy. However, the standard posterolateral thoracotomy and even a muscle-sparing thoracotomy are associated with significant acute and chronic pain as well as a lengthy recovery. It has been reasoned that a less invasive approach to lobectomy by the use of VATS techniques may be advantageous. It is hypothesized that the advantages of the VATS approach would stem from small incisions without transection or spreading of ribs, less postoperative pain, and a shorter hospital stay [1–7]. However, no uniform definition for VATS lobectomy has been accepted [8–11]. VATS lobectomy techniques can be describes as [1] mini-thoracotomy with video assistance; [2] simultaneously stapled lobectomy; [3] individual ligation lobectomy with a utility thoracotomy; and [4] a hybrid VATS lobectomy technique with features of isolation ligation and simultaneously stapled lobectomy [11].
One of the shortcomings of the VATS technique stems from the fact that the instruments are introduced through ports or small incisions which amount to holes in the chest wall. The instruments pivot at the entry hole and can be moved in four directions. The limited mobility of conventional endoscopic instruments, whether in the abdomen or the chest, has been referred to by some investigators as “chopstick surgery.” The chopstick nature and the limited maneuverability of the effector instruments stem from the rigid shaft axis fixed to the thorax by the entry hole. This technical shortcoming limits the surgeon in performing fine dissection and complex three-dimensional maneuvers. Pivoting instruments on the chest wall results in a large radius of curvature for the tip of the instrument and makes fine dissection in deep spaces such as the mediastinum very difficult and even dangerous. Indeed, it is this fact which has necessitated the need for a utility thoracotomy. Using the utility thoracotomy, the surgeon is able to utilize conventional instruments and his own wrist in order to provide additional degrees of freedom.
Another shortcoming of the VATS technique is in the lack of the three-dimensional visualization. The surgeon has to use two-dimensional information from the video monitor in order to create a three-dimensional mental image. This fact requires significant experience and can prove to be a source of fatigue for the surgeon. Most importantly, using such indirect means of judging depth perception is rarely equivalent to binocular vision. In the thoracic hilum, binocular vision is paramount for lymphadenectomy and individual vascular and bronchial dissection.
The use of the robotic technology obviates these difficulties. As has been pointed out in other chapters in this book, our view is that the daVinci Robot represents an ideal tool for dissection of the hilum and can be combined with VATS techniques to reach our ultimate goal of completely endoscopic lobectomy. In our view, the indispensable features of the daVinci Robot in performing VATS lobectomy are the following:
1.
The EndoWrist® (Intuitive Surgical, Mountain View, CA), or the cable-driven wrist at the end of the robotic arm: The placement of the robotic arm through the VATS hole is comparable to and accomplishes the chopstick maneuvers performed by conventional VATS instruments. However, the EndoWrist at the distal end of the robotic arm is positioned in the confined spaces within the chest and brings four more degrees of freedom and six additional directions of movement compared to normal VATS techniques. The EndoWrist provides the surgeon with fine instrument maneuverability in a very confined space.
2.
The daVinci Robotic system is designed to provide downscaling from the motion of the surgeon’s hand to that of the robotic instrument. This is invaluable in dissecting fine and fragile intrathoracic structures. Furthermore, a 6 Hz motion filter is used to filter out any tremor in the surgeon’s hand.
3.
The binocular robotic camera provides superb three-dimensional visualization, and by nature of being mounted on the central robotic arm, it can be manipulated by the surgeon. The surgeon’s ability to manipulate the camera and the arms recreates the natural biologic motion of the surgeon’s head, eyes, and hands in providing optimal hand-eye coordination.
Operative Technique
Anesthesia
Patients undergoing robotic-assisted VATS lobectomy require single-lung ventilation. We prefer a left-sided double-lumen endotracheal tube to a bronchial blocker. With a double-lumen tube, lung collapse is superior and hilar manipulation does not result in movement of the blocker and compromise in patient ventilation and surgical visualization. Longer tubing is required as the robot is brought in over the patient’s head and the anesthesiologist will occupy a more remote position than in usual video-assisted thoracic surgical procedures.
Patient Positioning
The patient is placed in a full lateral decubitus position. The table is flexed to open the intercostal spaces. The position of the double-lumen tube is reconfirmed after final patient positioning. The patient is then prepared and draped as in routine VATS procedures. The superior portion of the drape is allowed to cover the patient’s head. The table is unlocked and rotated 30° from its normal position in order to facilitate the positioning of the robot over the patient’s head.
Stage I: Routine VATS
The procedure is started using standard VATS instrumentation and incisions. The surgeon stands facing the patient’s back (Figs. 24.2 and 24.3). A 2 cm incision (camera incision) is made in the eighth intercostal space in the midaxillary line. A 10 mm metal trocar is introduced, and the Olympus EndoEYE (Center Valley, PA) video endoscope which is used in the VATS portion of the operation is positioned over the dome of the diaphragm.
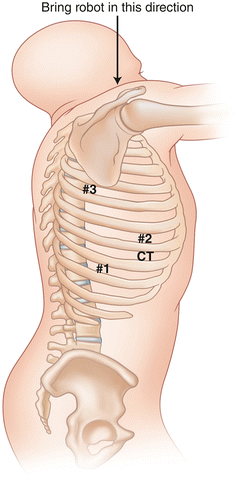
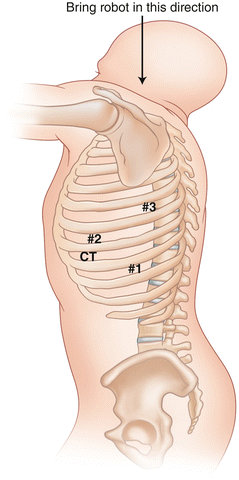

Fig. 24.2
Port placement for robotic lobectomy in the right chest

Fig. 24.3
Port placement for robotic lobectomy in the left chest
The oblique fissure is identified and an incision is made anteriorly on the chest wall overlying the oblique fissure in the anterior axillary line at least five fingerbreadths from the camera incision. This incision will be used for the left robotic arm during the robotic portion of the operation. A third 2 cm incision is made one interspace below the interspace for the anterior incision in the posterior axillary line at least five fingerbreadths from the camera incision. This incision will be used by the right robotic arm. It is important to note that as different pulmonary pathology results in changes in relationships between the fissures and the overlying intercostal spaces, the planning of our incisions is based on the initial view of the oblique fissure rather than an arbitrary predetermined positioning over certain intercostal spaces. This technique allows us to have the optimal visualization and access to the hilum in all patients. Furthermore, it is important to note that the anterior and posterior incisions need to be separated at least by the width of the surgeon’s hand in order to ensure optimal movement of the robotic arms. A fourth 1 cm incision is made in the same interspace and three fingerbreadths anterior to the camera incision. An atraumatic paddle retractor (Covidien, Inc. Minneapolis, MN) is introduced through this incision for retraction of the lung in an anterior and medial direction during robotic mediastinal dissection.
At the end of the procedure, a 28 Fr straight chest tube is introduced through this incision and positioned in the posterior aspect of the pleural space. The paddle retractor is used to sweep the lung medially and enhances the exposure of the mediastinum. Once the optimal positioning of the retractor is attained, it is held in place by a mechanical holder and fixed to the operating room table. For lobectomy procedures, the robot is positioned and repositioned two to three times depending on the lobe being resected.
Stage II: Robotic Dissection
1.
Mediastinal dissection: At this point, the robot is brought into the operating field over the patient’s head. The camera arm is introduced through the camera incision. In the right pleural space, the right robotic arm with the robotic hook cautery is positioned through the anterior incision and the EndoWrist is positioned over the mediastinum. The left robotic arm with a robotic DeBakey forceps is positioned through the posterior incision over the paddle retractor and its EndoWrist is also positioned over the mediastinum. A metal suction is introduced by the first assistant through the posterior incision below the robotic arm and is used to evacuate smoke, provide occasional retraction, and remove blood from the field. In the left pleural space, the hook cautery is introduced through the posterior incision and the forceps through the anterior incision. At all times the robotic arms are oriented such that the hook is manipulated by the surgeon’s right hand and the forceps by the surgeon’s left hand. At this juncture, the surgeon assumes his position at the robotic console. It is our preference for the surgeon to remain gowned and gloved such that he can rapidly attend to any unanticipated events without the need to rescrub. In the event of complication, the surgeon can remove the contaminated gown and glove, quickly change into a sterile gown and gloves, and attend to the patient in an expeditious manner. The pleura overlying the mediastinum is opened and all visible nodal tissue from the subcarinal and paraesophageal spaces as well as the pulmonary ligament area are removed at this portion of the operation. It is paramount to perform a complete mediastinal lymphadenectomy at this point of the lobectomy procedure. Nodes are placed in a small endobag and removed by the assistant at the table. Following the mediastinal dissection, the robotic camera and the robotic arms are repositioned by the surgeon to view the paratracheal region and a complete nodal dissection of the paratracheal region is completed. We find that the daVinci robot affords a deeper and more complete mediastinal lymphadenectomy as compared to the VATS technique and is comparable to an open procedure. Following the lymphadenectomy, the robotic arm and the camera are moved back to the posterior aspect of the hilum and used to dissect the proximal portion of the pulmonary artery, the anterior branch of the pulmonary artery, the bronchus, and the inferior pulmonary vein in cases which require a lower lobectomy.
2.
Anterior hilar dissection (upper lobectomy patients only): At this point, the surgeon changes his gown and gloves and the robotic arms are moved out of the chest. In patients undergoing upper lobectomy, paddle retractor is repositioned to retract the lung posteriorly and to expose the anterior aspect of the hilum. Once again, once optimal visualization is obtained, the paddle retractor is affixed to the table. The robotic camera is repositioned, this time to view the anterior hilum, and the right and the left arms are repositioned as described previously with similar instruments. However, this time, the EndoWrists are positioned over the anterior mediastinum. The robotic arms are then used to dissect the superior pulmonary vein and the anterior hilum.
3.
Pulmonary artery and dissection of the oblique fissure: As a third robotic maneuver in patients undergoing upper lobectomies or as a second robotic maneuver in patients undergoing middle or lower lobectomies, the robot is repositioned over the fissure. During this phase of the procedure, the N1 nodes are dissected and the pulmonary artery over its entire length is dissected and skeletonized.
A few principles are strictly adhered to in the robotic phase of the VATS lobectomy:
1.
A much wider dissection of the vascular and bronchial structures is performed. This allows for more mobility of the fragile structures and decreases the risk of catastrophic events.
2.
The robot is an excellent tool for dissection. The robotic platform is used for dissection of the fragile thoracic structures. Once the dissection is complete, we feel that manipulation and division of the vascular and bronchial structures are better accomplished using VATS instruments and stapling devices.
3.
In the event of bleeding, the assistant places pressure over the bleeding site with the metal suction. At this point, the robotic arms and camera are withdrawn and the control of bleeding is obtained using VATS techniques as have been described earlier in this chapter. After the bleeding is brought under control, the robot is repositioned and surgery continues. We have found the robotic platform to be unsuitable for control of bleeding or other catastrophic events. Once dissection is completed, the robot is withdrawn and lobectomy is completed by VATS. The vessels and bronchus are divided using conventional endoscopic staplers.
Following the completion of the lobectomy, the specimen is retrieved in the manner which was described earlier in this chapter through the anterior incision. The pulmonary vein stump is inspected for hemostasis and repaired with endoscopic suturing techniques using 4-0 Prolene® (Ethicon Endo-Surgery, Blue Ash, OH) if necessary. Sealant (Evicel®, Johnson and Johnson, New Brunswick, NJ) is applied to the staple lines as described earlier. The bronchial stump is tested with 30 cm airway pressure and the remaining lung is inflated under direct vision. A 28 French chest tube is positioned through the retraction incision as described earlier. The incisions are closed in two layers. The deeper muscle layer is closed with three separate simple sutures of 3-0 PDS. The skin and subcutaneous tissues are closed en bloc with three vertical mattress sutures of 2-0 Prolene. All patients undergo videobronchoscopy with inspection of the bronchial stump and removal of any obstructing secretions. All patients are extubated in the operating theater.
Endoscopic Robotic Esophagectomy
The history of esophageal surgery is a tale of men repeatedly losing to a stronger adversary yet persisting in this unequal struggle until the nature of the problems became apparent and the war was won. [14]
Although surgery has been the mainstay of treatment of esophageal carcinoma, the high morbidity and mortality rates associated with surgery have necessitated more palliative procedures as well as the search for nonsurgical therapies. Results of surgical resection have improved. In the 1960s and 1970s, the operability rate of esophageal carcinoma was 58 %, the resectability rate was 39 %, and the mortality associated with resection was 29 %, with an overall 5-year survival of 4 % [15]. In the 1980s, the resectability rate was 56 %, the mortality rate with resection was 13 %, and the 5-year survival was 10 % [16]. Presently in specialized centers which perform greater than 50 procedures per year, the mortality rate is reported at 4.5 % with a 5-year survival of 50.4 % overall [17]. Clearly this dramatic change in the overall survival and operative risk is due to the earlier diagnosis of esophageal carcinoma, refinement of surgical technique and perioperative care, and greater use of multimodality therapy.
Over the past decade, our program for esophageal carcinoma has followed the concept of en bloc resection of the tumor with extensive periesophageal lymphadenectomy. The en bloc resection of esophageal cancer has been accomplished using the Ivor-Lewis approach. In 2004, based on the Society of Thoracic Surgeons database, for patients undergoing esophageal resection using this technique at our institution, the mortality rate was 2 %, pneumonia was seen in 11 % of patients, and there were no anastomotic leaks.
This experience has formed the basis for robotic esophagectomy program at our institution [18]. At the outset of the robotic esophagectomy program, a number of parameters were identified:
1.
Our experience with VATS and laparoscopy had clearly demonstrated the feasibility of esophagectomy using minimally invasive techniques. However, in our experience the thoracoscopic approach impaired the ability to perform mediastinal nodal exoneration which has been a requirement of our conventional approach. The thoracoscopic approach was more akin to the lymphadenectomy that was achieved by the transhiatal esophagectomy.
2.
Posterior gastric dissection and celiac as well as mediastinal nodal exoneration were far less satisfactory with laparoscopy.
3.
The significant morbidity of conventional esophagectomy is due to the cervical esophagogastrostomy during the THE and the morbidity associated with a thoracotomy during the TTE. In order for the robotic program to obviate these difficulties, the thoracic portion of the procedure would need to be performed by minimally invasive techniques and the esophagogastric anastomosis needed to be positioned in the chest.
4.
The ideal robotic procedure would be one where a combined robotic video-assisted thoracoscopic and laparoscopic procedure would be accomplished with the placement of an esophagogastrostomy in the chest.
With the advent of the daVinci Robot, robotic surgical techniques have been applied to robotic laparoscopic mobilization of the stomach, transhiatal resection of the esophagus, and an open cervical esophagogastrostomy and robotic transthoracic mobilization of the esophagus combined with transhiatal esophagectomy and a cervical esophagogastrostomy. To the best of our knowledge, our program has been one of few centers which have emphasized video-assisted approach to esophagogastrostomy in the chest. It has been our contention that the addition of new robotic technology to the technique of transhiatal esophagectomy suffers from the shortcomings of the conventional THE.
Based on our experience with conventional esophagectomy, we determined that a robotic esophagectomy program should be designed based on the following parameters: (1) Oncologic efficacy of conventional procedures should be retained; (2) the complications associated with thoracotomy should be obviated; and (3) an intrathoracic esophagogastrostomy should be performed.
At the present time, the patients undergoing robotic esophagectomy at our institution undergo a transabdominal robotic laparoscopic mobilization of the stomach followed by a transthoracic robotic thoracoscopic esophagectomy and an intrathoracic video-assisted esophagogastrostomy.
Part I: Laparoscopic Gastric Dissection
The patient is placed in a supine position. Five abdominal ports are placed on the anterior abdominal wall, an approach similar to laparoscopic antireflux procedures. We prefer a 10–12 VersaPort™ (Covidien Autosuture™, Minneapolis, MN) for each of the trocar sites and Visiport™ (Covidien Autosuture™, Minneapolis, MN) instrument for entry into the peritoneum. The peritoneum is entered below the umbilicus using the Visiport instrument. The abdomen is inflated, and ports #2 and #3 are placed at the level of the umbilicus at the right and left mammary lines. A Diamond-Flex® (Snowden Penser®, Tucker, GA) liver retractor is introduced through port number two and placed under the left lateral segment of the liver. The retractor is fixed by a self-retaining system (Mediflex, Velmed, Wexford, PA) to the operating table. This maneuver exposes the esophageal hiatus. An endoscopic Babcock is introduced through port #3 and used to retract the stomach inferiorly. Ports #4 and #5 are placed in the subcostal region and are positioned to “line up” with the left and right sides of the esophageal hiatus. The phreno-esophageal ligament is opened using Endoshears and the esophagogastric junction is exposed. The Harmonic® Scalpel (Ethicon Endo-Surgery, Blue Ash, OH) is used to divide the gastrocolic omentum and the short gastric vessels. The gastroepiploic artery is preserved. The endoscopic Babcock is removed and a fan retractor (Auto Suture, U.S. Surgical Inc) is positioned through incision #3 and the stomach is retracted superiorly. The left gastric pedicle is divided using an endoscopic reticulating 45 mm vascular stapler (Ethicon Endo-Surgery, Blue Ash, OH). We generally do not perform a pyloromyotomy or pyloroplasty during the laparoscopic procedure. The occasional postoperative pyloric obstruction has been shown to be easily amenable to balloon dilatation. The gastric tube is constructed by dividing the stomach using an EZ 45 stapler (Ethicon Endo-Surgery, Blue Ash, OH) starting just proximal to the right gastric artery on the lesser curve of the stomach and proceeding to the apex of the greater curve. Due to the greater ease of suturing in the chest, the gastric suture line is oversewn using 000 polypropylene suture during the robotic video-assisted portion of the procedure. The esophagus is divided using the EZ-45 stapler at just above the gastroesophageal junction. Using an endostitch with 0 Ethibond (Ethicon Endo-Surgery, Blue Ash, OH) suture, the proximal gastric tube is attached to the transected distal esophagus. The jejunostomy is fashioned by suturing a loop of proximal jejunum onto the anterior abdominal wall with an endostitch instrument and 00 Ethibond suture. A needle catheter jejunostomy kit (Compact Biosystems, Minneapolis, MN) is used to percutaneously place a jejunostomy catheter into the jejunum. The jejunal entry site is further fixed to the anterior abdominal wall in order to prevent spillage and torsion. At the conclusion of the procedure, the right and left sides of the right crural arch are sutured using an endostitch instrument with 0 Ethibond suture. This maneuver facilitates the suture ligation of the phrenic vein which overlies the crural arch. The crural arch is opened with Endoshears™ (Covidien, Minneapolis, MN) and the distal esophagus is further mobilized. This maneuver invariably results in loss of pneumoperitoneum and should be reserved for the conclusion of the procedure. The trocar sites are closed using an EXIT instrument and 0 Vicryl (Ethicon Endo-Surgery, Blue Ash, OH). Subcutaneous tissues are closed with 00 Vicryl® and the skin is closed with staples. In these patients esophagectomy and an intrathoracic esophagogastrostomy are accomplished using robotic right video-assisted thoracic surgical techniques.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








