Fig. 48.1
Patient undergoing abdominal wall reconstruction with Alloderm™ (LifeCell, Branchburg, NJ) who has undergone full vascularization of the biologic graft. (Reproduced with permission from Latifi R, Peralta R, Al Thani H. Abdominal Wall Reconstruction in Patients with an Open Abdomen and Enterocutaneous Fistulas: A 9 Step Treatment Strategy. In Latifi R (ed): Surgery of Complex Abdominal Wall Defects. New York: Springer Science + Business Media; 2013)
The aim of this chapter is to review current clinical application of biologic mesh used for abdominal wall reconstruction [13] and few other indications currently being used.
Abdominal Wall Defect and Enterocutaneous Fistulas
The need for major abdominal wall reconstruction has expanded. Once used mainly for trauma patient, who have undergone damage control (DC) operation, now is being used more and more in acute catastrophic abdominal conditions such as perforated viscus, grossly contaminated abdomen, when abdomen cannot or should not be closed, and whom are managed by open abdomen. However, while DC has been shown to save lives, its liberal use has it consequences and has been called an “overused procedure” [14], despite the fact that it has been accepted around the world. Higher medical cost, enterocutaneous fistulas (ECFs), and inability to be fully functional due to massive ventral hernias for long periods of time remain the most serious complications of open-abdomen management techniques and damage-control surgery, particularly in acute care and trauma surgery [15–17]. ECFs are associated with significant morbidity and mortality despite significant advances in surgical techniques and technologies for patients with complex abdominal wall hernias. Seventy-five to eighty-five percent of ECFs are postoperative and most patients with ECFs also have abdominal wall defects (through which the ECFs become evident); therefore, it is important that surgeons treat both conditions in tandem. Especially challenging is the combination of ECFs and any or all of these conditions: large abdominal defects, an open abdomen, entero-atmospheric fistulas (EAFs), or stomas. The incidence of ECFs in combination with an open abdomen on the other hand has been reported to be as high as 75 % [3]. While closing the open abdomen and establishing functional abdominal wall in these patients, especially in those with fistulas and stomas, represents a major challenge and requires surgical creativity, deciding of what kind of mesh to use is also a major decision. Multidisciplinary approaches and advanced surgical techniques are required. Whatever technique is used, the goal is to create coverage of the abdominal cavity and to improve the patient’s quality of life. Native abdominal wall can be used; if that is not possible, biologic or prosthetic mesh can be used instead. In most patients, some sort of combination of reconstruction techniques will be needed. If native tissue can be used without undue tension, then it should be used; but if midline tissue cannot be easily approximated or if mesh reinforcement is needed (as it is in almost all abdominal wall defects larger than 6 cm), creative techniques must be considered. In addition to the component separation technique that is based on an enlargement of the abdominal wall surface by separating and advancing the muscular layers (Fig. 48.2), there will be a need for a mesh re-enforcement or entire reconstruction using a mesh.
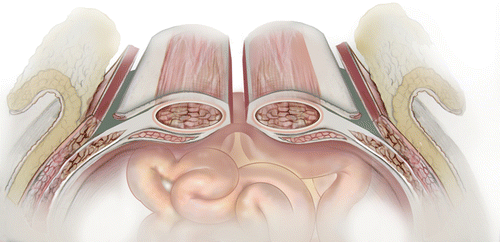

Fig. 48.2
Illustration of lateral component separation. (Used with permission from LifeCell Corporation, an Acelity Company)
By definition, patients with ECFs, EAFs, and/or stomas have contaminated wounds. Synthetic mesh has been used in the past, but it was associated with high rates of wound infection (often necessitating removal of infected mesh for source control of infection) as well as other complications (such as newly created fistulas). The fear of infection when using synthetic mesh has been a major facet of the decision to use biologic mesh instead.
The most common application of bioengineered tissue, created, or modified is in general surgery. Reconstruction of major abdominal walls with modified tissues that is harvested from human or pigs has become a common practice. In an experimental study examining biologic grafts in comparison to synthetic material, biologic grafts are able to clear a Staphylococcus aureus contamination; however, they do so at different rate [18]. The authors created a chronic hernia model in rats and then used various meshes (one synthetic polyester as control material (n = 12) and four different biologic grafts (n = 24 per material)). Biologic grafts evaluated included Surgisis® (Cook Medical, Bloomington, IN) (porcine small intestinal submucosa), Permacol™ (Covidien, Minneapolis, MN) (crosslinked porcine dermis), Xenmatrix™ (CR Bard, Murray Hill, NJ) (noncrosslinked porcine dermis), and Strattice™ (LifeCell, Branchburg, NJ) (noncrosslinked porcine dermis). Half of the repairs in each group were inoculated with Staphylococcus aureus at 104 CFU/ml and survived for 30 days without systemic antibiotic. There was a significant difference of bacterial clearance between biologic meshes. To this end, use of biologic mesh in Northern America has become standard in high-risk patients with contaminated and dirty-infected wounds, despite the very high cost associated with the use of biologic mesh.
Previously we reported our own study of 60 patients who underwent acellular dermal matrix (ADM) implantation for abdominal wall reconstruction [19]. Of the 60 patients, 56 patients had enough information on their charts and were studied retrospectively. In these patients we used two brands of ADM: AlloDerm® (LifeCell, Branchburg, NJ) in 38 patients (68 %) and Strattice™ (LifeCell, Branchburg, NJ) in 18 (32 %). A total of nine patients had concomitant ECFs and/or EAFs. For the nine patients with ECFs and/or EAFs, we used underlay placement in 4 (44 %) and interposition or bridge placement in 5 (56 %). We found that the abdominal wall reconstruction results in patients with vs. without concomitant ECFs and/or EAFs did not statistically differ, in terms of the rates of overall complications, of recurrence, and of infectious complications. However, we lack long-term data on these patients.
Others have also reported that ADM implantation can be safely used to repair large and complex ventral hernia defects in patients with clean-contaminated or dirty-infected wounds. In our study mentioned above, of the 56 patients who underwent ADM implantation with either AlloDerm or Strattice, 35 had contaminated fields as defined by the presence of intra-abdominal or soft tissue infections, stomas, or fistulas. Of those 35 patients, the majority—26 (74 %)—had Grade 4 infections, per a hernia grading system [20]. The grading system, which was recently created, is used to classify the risk for infectious complications in order to help surgeons decide on the technique and potentially on the mesh to be used. Grade 1 refers to a low risk for infections or complications in patients who have no history of wound infections; Grade 2 refers to comorbidities such as smoking, diabetes, obesity, a suppressed immune system, and chronic obstructive pulmonary disease (COPD); Grade 3 refers to previously contaminated wound infections, stomas, or intraoperative violations of the GI tract; and Grade 4 refers to infected mesh and septic foci. Obviously, Grades 3 and 4 present serious medical and surgical challenges for the patient and for the health care team, whether led by a general surgeon, trauma surgeon, or plastic surgeon but even Grade 2 means that patients may harbor a significant risk and need to be thoroughly evaluated preoperatively; otherwise a significant problem could arise. My own preference on this issue is to actually review the patient’s history carefully and if possible, to use synthetic mesh unless the patient clearly is a Grade 2 with a high risk for infection and is undergoing major ventral hernia repair.
Our results suggest that biologic mesh implantation is a valid option for complex abdominal wall reconstruction in the high-risk trauma and acute care surgery population; however, long-term results are not evident yet. Other surgeons have reported abdominal wall closure in the infected field as well. In a recent study of 82 patients [21] with ventral hernia repaired predominantly with Alloderm and Strattice, 32 (39 %) had had concomitant bowel surgery. There was no difference in hernia recurrence (contaminated group—28 % vs. noncontaminated group—34 %, P = 0.58), surgical site infections (contaminated—28 % vs. noncontaminated—20 %, P = 0.40), or other complications when patients with and without concomitant bowel surgery were compared. One of the major complications of the biologic mesh has been hernia recurrence rate, which has been reported as high as greater than 30 % [22] or laxity that troubles both the patient and the surgeons [23]. In this study, seven of the nine patients reconstructed with component separation followed by interpositional Alloderm presented with abdominal wall laxity. Laxity was defined as a condition in which patients had clinical evidence of abdominal bulge at follow-up and required secondary reconstruction. While laxity has been common in our own patients as well, long-term data are missing [17]. In a systematic review of 25 retrospective studies performed by Slater et al. [24], the authors found that recurrence rate depended on wound class, with an overall rate of 13.8 % (95 % confidence interval [CI], 7.6–21.3). The recurrence rate in contaminated/dirty repairs was 23.1 %. Abdominal wall laxity occurred in 10.5 % of patients. The surgical morbidity rate was 46.3 %. Infection occurred in 15.9 % of patients but only led to graft removal in 4.9 % of cases. As it has been known for a while now, there is no randomized clinical trial; however, biologic grafts are associated with a high salvage rate when faced with infection. Use of biologic mesh has made possible “one operation only” as it is attempted by most surgeons who perform abdominal wall reconstruction at the time of hernia repair or at the time of takedown of ECFs and/or EAFs, even in contaminated fields, instead of series of operation. In our practice, we aim to complete the definitive procedure in a single operation. On occasion, I have used the principle of damage control, returning the next day or so to complete the operation by performing anastomosis, preparing the abdominal wall by performing lateral compartment release, and returning the next day for mesh placement and final closure.
In this study of 128 patients (76 F, 52 M) with large hernia defect (range 40–2,450 cm2), infected mesh was present in (n = 45), stoma (n = 24), concomitant gastrointestinal (GI) surgery (n = 17), enterocutaneous fistula (n = 25), open nonhealing wound(s) (n = 6), enterotomy/colotomy (n = 5), and chronic draining sinus (n = 6) [22]. Despite the high rate of wound morbidity (47.7 %) associated with single-staged reconstruction of contaminated fields, authors concluded biologic mesh can be placed without consequences. However, these authors also conclude that the long-term durability seems to be less favorable. In a similar study, this group of authors reported the simultaneous reconstruction of ECF and complex abdominal wall defects resulted in successful single-stage management of these challenging cases in nearly 70 % of patients [25]. To this end, many authors now believe that abdominal wall reconstruction (AWRs) using ADM has low rates of surgical site occurrence (SSO) and surgical site infection, despite increasing degrees of wound contamination. Most recent study of 359 patients who had AWR using ADM, with mean follow-up of 28.3 ± 19.0 months, further supports his hypothesis [26] irrespective of what kind of approach was used and despite all the advances in mesh and in techniques used, the AWR with or without biologic mesh has very high morbidity. In a report of 106 patients [27] (79 patients of whom had preoperative comorbid conditions), 67 (63 %) patients developed a postoperative complication. Skin necrosis was the most common complication (n = 21, 19.8 %). This is similar to our experience [28]. This is particularly common with component separation and if the patient undergoes massive resuscitation. Other complications of AWRs include seroma (n = 19, 17.9 %), cellulitis (n = 19, 17.9 %), abscess (n = 14 13.2 %), pulmonary embolus/deep vein thrombosis (n = 3, 2.8 %), small bowel obstruction (n = 2, 1.9 %), and fistula (n = 8, 7.5 %). Using the Methodological Index for Non-randomized Studies and those with a score of 8 or more were combined to evaluate the end points of 16 studies from six different mesh products, a most recent study [29] analyzed 554 patients with an overall infection rate of 24 % and a recurrence rate of 20 %. The authors call for caution when using biologic mesh products in infected fields because there is a paucity of controlled data and none have US Food and Drug Administration approval for use in infected fields. When biologic mesh was compared to nonbiologic mesh in a recent meta-analysis, it was found that biologic grafts had significantly fewer infectious wound complications (p < 0.00001); however, recurrence rates were not different. In addition, there were no differences in wound infections, and recurrence between the human and porcine-derived biologic grafts [30].
Graft Placement
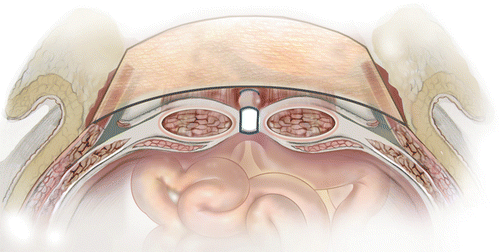
Either open or laparoscopic surgical techniques can be used to repair abdominal wall defects; but, in patients with ECFs and/or EAFs, the open approach is preferred. There are three most common techniques used to place mesh during abdominal wall reconstruction, and these are onlay placement, underlay placement, and interposition or bridge placement.
Onlay Placement
Technically, onlay mesh placement is the easiest way to place mesh. I used this technique (Fig. 48.3) at the beginning of my practice and still use it on occasions when we are able to approximate the abdominal wall edges without any major dissection or if I use synthetic mesh. The key element of this approach is fixing the mesh both laterally and over the edge of midline. I prefer fixing mesh to fascia using absorbable sutures, either interrupted or in a continuous fashion. The main objective is to reestablish tight approximation of the mesh to fascia. In addition, I use three or four large, closed suction drains (19 French [Fr]) under the subcutaneous tissue and keep them in until the individual drain output is less than 25 cc over 24 h.