Fig. 39.1
Toyoda’s commissuro-annuloplasty technique for performing bench tricuspid annuloplasty for donor hearts at the back table (courtesy of Angelo Rutty, MD.)
Primary graft failure remains the most common cause of death within 30 days following heart transplantation and accounts for approximately 40 % of the 30-day mortality. Right ventricular failure is the most common type of primary graft failure after heart transplantation because of the unique circumstances after heart transplantation. These circumstances include high afterload for the right ventricle and myocardial injury. Heart transplant recipients tend to have high pulmonary vascular resistance due to long-standing heart failure. The majority of the patients have risk for bleeding such as multiple sternotomy, standing anticoagulation medication such as warfarin, clopidogrel, and aspirin, mechanical circulatory support causing coagulopathy, and severe heart failure. Increased usage of blood products, platelets, red blood cells, etc. and medications such as protamine and vasoconstrictors such as norepinephrine and phenylephrine can elevate pulmonary vascular resistance. The allograft ischemic time usually ranges between 1 and 6 h and ischemia/reperfusion injury causes myocardial damage leading to ventricular failure. In addition, acute/hyper acute rejection, air embolization, etc. can also cause ventricular dysfunction. In such situations, the right ventricle dilates according to the Frank-Starling law in order to maintain cardiac output. The right ventricular dilation results in tricuspid valve annular dilatation leading to significant tricuspid valve regurgitation. Severe tricuspid regurgitation can further dilate the right ventricle. Thus, the vicious cycle starts and eventually results in right ventricular failure. Moreover, high central venous pressure caused by tricuspid valve regurgitation can cause renal failure leading to volume overload. The prophylactic “commissuro-annuloplasty” prevents development of right ventricular failure because of the mechanism shown in Fig. 39.2. This effectively reduces the tricuspid annular size by 2–4 cm, preventing annular dilatation in the setting of right ventricular dilatation and improving coaptation of the leaflets preventing significant tricuspid regurgitation.
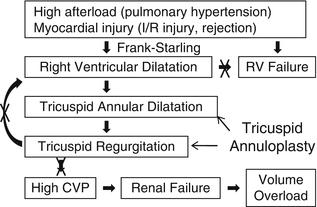

Fig. 39.2
Mechanism of right ventricular failure and effect of tricuspid annuloplasty after heart transplant
Case 1
The recipient is a 63-year-old male with ischemic cardiomyopathy. The donor was a 25-year-old male with transportation time of 3 h and 15 min. The donor died of head trauma. The echocardiography showed mild to moderate tricuspid valve regurgitation without evidence of leaflet or chordal disease. Orthotropic bi-caval heart transplantation with a donor tricuspid annuloplasty at the back table was performed with total allograft ischemic time of 4 h and 15 min and cardiopulmonary bypass time of 2 h and 20 min. A tricuspid annuloplasty was performed using two pledgeted 3-0 Prolene to plicate the posterior annulus. The recipient had uneventful postoperative course. Postoperative echocardiography at weeks 1 and 2 showed no tricuspid regurgitation and at 4 months, there was trace tricuspid valve regurgitation.
Tricuspid Valve Surgery After Heart Transplantation
There are two structural mechanisms of tricuspid regurgitation, namely normal leaflet motion with annular/ventricular dilation and excessive leaflet motion [3, 4]. Possible mechanisms include right ventricular dilatation due to elevated pulmonary vascular resistance [3, 4], acute allograft edema, which diminishes with time [16], papillary muscle dysfunction [17], disturbed geometry of the right atrial anastomosis with subsequent impairment of the functional integrity of the valvular apparatus, cyclic torsion of the atria during ventricular systole and diastole, asynchronous contraction of the donor and recipient atrial compartment in the bi-atrial technique [18], and biopsy-induced flail tricuspid valve [19]. The indication for tricuspid surgery is severe tricuspid valve regurgitation and clinical signs of right heart failure with fatigue, decreased exercise tolerance, hepatomegaly, and lower extremity edema [20, 21]. Common repair techniques include annuloplasty with bicuspidization, DeVega and ring and bands, artificial chordoplasty for prolapse, valve excision for endocarditis, and patch-plasty for perforation of the leaflet. Valve replacement is performed with bioprosthesis; mechanical prosthesis should be avoided due to necessity of endomyocardial biopsies.
Authors’ Recommendations
1.
Bench tricuspid annuloplasty should be considered under the following situations:
(a)
Preoperative echocardiography at the donor hospital shows mild or greater tricuspid regurgitation.
(b)
The recipient’s pulmonary vascular resistance is markedly elevated, e.g., trans-pulmonary gradient greater than 15 mmHg.
2.
Suture annuloplasty with the author’s technique is the preferred method for bench annuloplasty because it can be done very quickly without significantly prolonging allograft ischemic time and the annuloplasty needs to be effective only during immediate postoperative period when the recipient pulmonary vascular resistance remains high and the tricuspid regurgitation is most prevalent.
3.
When the patient comes off cardiopulmonary bypass, annuloplasty should be considered for cases with moderate to severe tricuspid regurgitation. In this situation, the allograft is already reperfused and repair needs to be performed through a right atriotomy; therefore, appropriate surgical techniques such as ring annuloplasty should be chosen for a durable repair.
4.
Late tricuspid regurgitation: Tricuspid valve repair utilizing ring annuloplasty and/or artificial chordoplasty is indicated for moderate to severe tricuspid regurgitation with hemodynamic compromise and symptoms of right heart failure.
Mitral Valve Surgery in Heart Transplantation
Mitral Valve Surgery at the Time of Heart Transplantation
To expand the cardiac donor pool, mitral valve surgery has been performed to repair significant donor mitral valve disease at the time of heart transplant [22–24]. Bench ring annuloplasty has been reported for tricuspid regurgitation with normal leaflet motion and annular dilation [22, 23]. Bench commissurotomy and DeVega annuloplasty have also been reported for organic mitral valve disease due to rheumatic process [24]. This author prefers to perform bench mitral annuloplasty for donor hearts at the back table using Kay-Reed annuloplasty [14, 15] only when the echocardiography at the donor hospital shows mild or greater mitral regurgitation before procurement of the heart.
Case 2
The recipient was a 56-year-old male with ischemic cardiomyopathy. He was in UNOS I-A with 0.75 mcg/kg/min of milrinone and 3 mcg/kg/min of dopamine and supported by the intra-aortic balloon pump in the intensive care unit. His right heart catheterization showed pulmonary artery pressure of 77/40(54), pulmonary artery wedge pressure of 41 mmHg, and trans-pulmonary gradient of 13 mmHg. The donor was a 23-year-old male with the transportation time of 1 h and 45 min. He died of a motor vehicle accident and suffered from severe blunt chest trauma with elevated myocardial enzyme level. The echocardiography showed mild to moderate mitral valve regurgitation without evidence of leaflet or chordal disease. An orthotopic bi-caval heart transplantation with a donor mitral annuloplasty at the back table was performed with total allograft ischemic time of 2 h and 44 min and cardiopulmonary bypass time of 2 h and 17 min. The mitral valve was tested with injection of cold saline in the left ventricle, which showed leakage through the posteromedial commissural. A mitral annuloplasty was performed in Reed’s technique using 1 pledgeted 3-0 Prolene to plicate the posteromedial commissure. The patient had uneventful postoperative course and is doing well. Postoperative echocardiography at week 2 showed trace mitral valve regurgitation. Right heart catheterization 8 months postoperatively showed pulmonary artery pressure of 25/4 (15) mmHg, and pulmonary artery wedge pressure of 9 mmHg with cardiac index of 3.30 l/min/m2.
Mitral Valve Surgery After Heart Transplantation
Although significant mitral valve disease after heart transplantation is rare, the need of mitral valve surgery upon transplanted hearts is predicted to become more common with increasing longevity of heart transplant recipients and with efforts aimed at preserving cardiac allograft function to avoid cardiac re-transplantation since the donor shortage is the major limiting factor of heart transplantation [25, 26]. Unlike coronary arteries, the long-term function and structure of mitral valve has been shown to be preserved [27]; however, mitral valve repair and replacement have been reported upon transplanted hearts. Mechanisms of mitral insufficiency include left-sided endomyocardial biopsy, endocarditis, myxomatous degeneration, fibrosis of papillary muscles, and chordae tendineae due to rejection, allograft coronary artery disease leading to ventricular dysfunction with mitral annular dilation, and potential distortion of the mitral valve structure due to left atrium-to-atrium anastomosis [25–27]. Mitral valve surgery after heart transplantation can be performed with conventional techniques, indications, and outcomes.
Aortic Valve Surgery in Heart Transplantation
Aortic valve surgery is rare in relation to heart transplantation; however, in efforts to expand the donor pool, donor hearts with significant aortic valve disease can be utilized. Bench repair of donor aortic valve with sub-commissural annular plication has been performed for moderate central aortic insufficiency with normal valve structure [28]. Others reported aortic valve replacement for significant aortic insufficiency and valve-sparing aortic root replacement with reimplantation technique for type A aortic dissection with significant aortic insufficiency after heart transplantation [29, 30]. Unique to heart transplantation, distortion of the donor ascending aorta can cause severe aortic regurgitation requiring aortic valve replacement [31]. Thus, aortic valve surgery can be performed using conventional techniques, indications, and outcomes in relation to heart transplantation.
Valve Surgery in Lung Transplantation
Valve Surgery at the Time of Lung Transplantation
Over the last decades, lung transplantation has become a viable treatment for a variety of end-stage lung disease with improved outcome due to better surgical techniques, organ preservation against ischemia/reperfusion injury, immunosuppression against rejection, and infection prophylaxis [32]. Currently, the major limiting factor is the shortage of donor organs. To maximize utilization of donor organs for lung transplantation, this author has recently reported how to harvest the donor lungs from patients who had undergone cardiac valve surgery [33]. Traditionally, patients with other significant comorbidities including cardiac diseases have been denied for lung transplant candidacy [3]. With the improved outcome in lung transplantation, however, a few centers have performed lung transplantation concomitant with cardiac surgery including coronary artery bypass, patent foramen ovale closure, atrial septal defect repair, ventricular septal defect repair, patent ductus arteriosus repair, replacement of the ascending aorta, and DeVega tricuspid valve repair [34–36]. Thus, in selected cases, cardiac problems including valvular heart diseases can be repaired at the time of lung transplantation.
Tricuspid regurgitation is the most common valvular disease in lung transplant recipients because patients frequently have pulmonary hypertension due to end-stage lung disease, and this elevated afterload makes the right ventricle dilated, resulting in tricuspid annular dilatation. However, whether we should operate on the tricuspid valve or not and when we should repair it are unknown at the time of lung transplantation. A group from Austria has reported that tricuspid regurgitation disappeared 3 months after double-lung transplantation in patients with primary pulmonary hypertension due to reverse remodeling of the right ventricle with normalized or reduced pulmonary vascular resistance [37]. Another group has also reported similar results after single-lung transplantation for pulmonary hypertension [38]. However, the mortality rate at 3 months after lung transplantation was 17.5 % [37], and the most recent International Society of Heart and Lung Transplantation Registry data show that primary pulmonary hypertension has the lowest survival immediately after lung transplantation in the major diagnostic categories with initial evident separation in survival between primary pulmonary hypertension and chronic obstructive lung disease and cystic fibrosis [32]. The registry speculates that this initial difference in survival may be related to differences in the complexity of the surgery and the frequency and severity of early complications [32]. This author speculates that this high early mortality in primary pulmonary hypertension is related to heart failure, predominantly right heart failure with tricuspid regurgitation leading to multiple organ failure. It is well documented that right heart failure manifests as enlarged right atrial size due to high right atrial pressure and the severity of tricuspid regurgitation predicts mortality in primary pulmonary hypertension [39]. Severe tricuspid regurgitation is the indication for repair or replacement to improve survival either as an isolated disease or as functional tricuspid regurgitation associated with left-sided valvular diseases [40]. In this author’s institution, tricuspid valve repair concomitant with double-lung transplantation is the procedure of choice for patients with primary pulmonary hypertension with severe tricuspid regurgitation, and with this strategy for primary pulmonary hypertension, from 1994 to 2006, the institution’s survival rate is 86 % at 1 year, 75 % at 5 years, and 66 % at 10 years, which is better by 20–39 % compared to the 23rd official report from the registry of the International Society of Heart and Lung Transplantation data from 1994 to 2004, which is 66 % at 1 year, 39 % at 5 years, and 27 % at 10 years [41]. This author believes the importance of tricuspid valve repair at the time of lung transplant for severe tricuspid regurgitation in improving early survival after lung transplantation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








