CHAPTER 76
Surgical Treatment of Trigeminal Neuralgia
HISTORY
Facial pain can be caused by a variety of factors, including but not limited to dental pathology, trauma, multiple sclerosis, tumors or other brain lesions, temporomandibular joint (TMJ) disorders, and myofascial and psychogenic disorders. Although most of these are difficult to treat with either medical or surgical therapy, it is possible to address the underlying insult (tumor removal, resolving dental pathology, resting the TMJ with a bite block) and improve pain by eliminating the offending agent. Trigeminal neuralgia (TM) is a specific type of facial pain syndrome which can usually be clinically distinguished from these other disorders. Proper recognition of the disorder and accurate diagnosis of TN is critical, since there are numerous therapeutic options that are effective for TN but these are of limited or no clear utility for most of the other forms of facial pain. The first known report of symptoms typical of TN came from the famous physician John Locke in 1677.1 The patient described typical severe, sharp, unilateral pain of the face and lower jaw. Interestingly, the patient had teeth removed without relief. This continues to occur to this day, since minor tooth pathology may lead to dental procedures or extractions in a somewhat speculative attempt to treat facial pain that in fact is TN. Nicolas Andre coined the term “tic douloreux,” another term still in use which describes the facial contraction and contortions that often accompany the paroxysmal pain episodes of TN.2 Until early in this century, TN was still believed to involve the facial nerve because of these often seen contractions, but it has long been established that this is simply a reactive muscular contraction during the severe pain episodes.
DIAGNOSIS
• TN is usually characterized by unilateral, paroxysmal sharp or burning pain of brief duration (seconds to minutes) in either the maxillary (V2) and/or mandibular (V3) distributions of the trigeminal nerve beginning in patients roughly 50 to 70 years of age.
• Involvement of the ophthalmic (V1) division, bilateral symptoms, and younger age of onset can happen but are rare and should suggest consideration of an alternative diagnosis.
• Undiagnosed multiple sclerosis can present with TN-like symptoms, and very young age of onset (20-40-year old) should promote further testing for this possibility.
• The additional presence of more chronic, less intense, and less sharp pain does not preclude a diagnosis of TN as long as the other type of pain is present, and this type of pain may in fact be due to the severe facial contractions that occur in reaction to TN episodes.
• TN patients can often have periods of remission, which in some cases can last for years. However, recurrence is common, and generally any subsequent remission periods are of increasingly shorter duration.
• There is also often a specific “trigger” zone, which can bring on a pain episode when stimulated. Stimuli which are frequently reported as provoking TN symptoms include chewing, brushing teeth, rubbing the area of the trigger, and wind brushing across the face.
PATHOPHYSIOLOGY
• Eminent neurosurgeon Walter Dandy in 1934 noted during surgery to partially section the trigeminal nerve that the nerve is frequently indented or lifted up by an artery, and he specifically speculated that this was the cause of TN.
• The primary cause of most cases of classic TN is believed to be compression of the trigeminal nerve by an arterial loop, usually from the superior cerebellar artery.
• On occasion, open surgical intervention (described in detail below) fails to demonstrate a clear offending artery, but large veins have been noted to be touching the nerve.
• Whether venous compression can cause trigeminal neuralgia is unclear but this has been discussed.
• This presumably developmental variant causing onset of symptoms so much later in life is unclear, but it may be that either volume loss with age causes slight brain shifts that bring cranial nerves in closer proximity to vascular structures and/or pulsations over many years leads to demyelination or other alterations in the nerve which eventually facilitates the pain response.
MEDICAL THERAPY
Medical therapy for TN is based on the belief that the progressive irritation from vascular compression leads to inappropriate firing of the nerve and the resulting spasmodic pain. Therefore, as with other forms of neuropathic pain, the primary medications utilized in TN are anticonvulsants and antidepressants. Carbamazepine is frequently utilized and was recently endorsed in a consensus statement of the American Academy of Neurology as having established efficacy based upon the strength and number of class I and class II studies. This can influence white blood cell counts and therefore requires regular monitoring. Oxcarbazepine is also often utilized and there are several strong clinical studies in support of this treatment as well. Most other anticonvulsant or neuropathic pain medications, baclofen, and antidepressant medications have all been utilized with symptomatic relief in patients, but these have been less rigorously tested in high-quality clinical trials.
Problems With Medical Therapy
• Limited efficacy in many patients.
• Dose-limiting adverse effects and disease progression/resistance to therapy.
• More severe cases may have limited responses to medication which are inadequate to permit a reasonable quality of life.
• Some patients who have adequate pain relief cannot tolerate the medications due to adverse effects at doses necessary for pain control.
• These adverse effects are usually a consequence of the mechanism of medication action, which treat TN by reducing firing of the hyperactive trigeminal nerve.
• It also can similarly reduce activity of neurons throughout the brain.
• Therefore, patients can complain of difficulty with concentration or focus, excessive lethargy or sleeping, and personality changes.
• Mild side effects often resolve spontaneously but more significant complications can severely limit the effectiveness and utility of medical therapy.
• Patients who may have responded to medication for months or even many years can develop worsening pain over time either due to decreased response to medical therapy or doses can no longer be safely increased.
SURGICAL THERAPY
Several effective surgical options are available to patients who have inadequate responses to more conservative therapy. Given the high response rates to some of the surgical procedures outlined below, it is not entirely clear if exhausting all medical therapies is appropriate management of patients with typical trigeminal neuralgia. Nonetheless, understandable fears of surgery and potential risks of surgery generally lead patients and caregivers to attempt medical therapy initially, and therefore most patients have at least attempted medical management prior to considering surgical intervention. Most of the current surgical therapies have been available for several decades or more, and therefore there is a large and long experience to help provide patients with confidence regarding the known effectiveness and risks of each procedure. However, with the use of neurostimulation for other types of pain, this is now being explored in mostly off-label or experimental fashions and may also provide a more technologically advanced alternative, particularly in patients who have either atypical syndromes or who have not adequately responded to traditional surgical treatments.
MICROVASCULAR DECOMPRESSION
Microvascular decompression (MVD) is the most invasive of all surgical options for TN, but it also appears to have the best therapeutic outcomes in most series. Roughly 90% of patients are either pain free or have dramatic pain relief soon after surgery, with more than 70% of patients still reporting absence of pain at 5 years in most series. The reason for such strong therapeutic efficacy is likely because MVD is the one procedure which directly addresses the presumptive pathology causing TN.
• This is an open microsurgical procedure in which a retrosigmoid craniotomy is performed just up to the junction between the transverse and sigmoid sinuses in order to expose the trigeminal nerve.
• The cerebellum is retracted to expose the nerve, although significant drainage of spinal fluid helps to minimize the amount of retraction needed.
• After the nerve is identified, it must be explored throughout its entire traverse in the subarachnoid space, extending from Meckel cave laterally to the brainstem root entry zone medially.
• Often petrosal vein branches are present in the field, and these can be coagulated and divided to prevent hemorrhage and facilitate decompression.
• An artery (most commonly the superior cerebellar artery) is usually identified as indenting the nerve, most commonly near the root entry zone but this can occur anywhere along the intracranial expanse.
• With modern imaging, this artery can often be observed on preoperative MRI (Figure 76-1A), and these intraoperative findings can closely mimic imaging findings (Figure 76-1B). The artery is then mobilized away from the nerve and then a Teflon-coated cotton pad is placed in between the nerve and artery to cushion the nerve and relieve the compression (Figure 76-2).
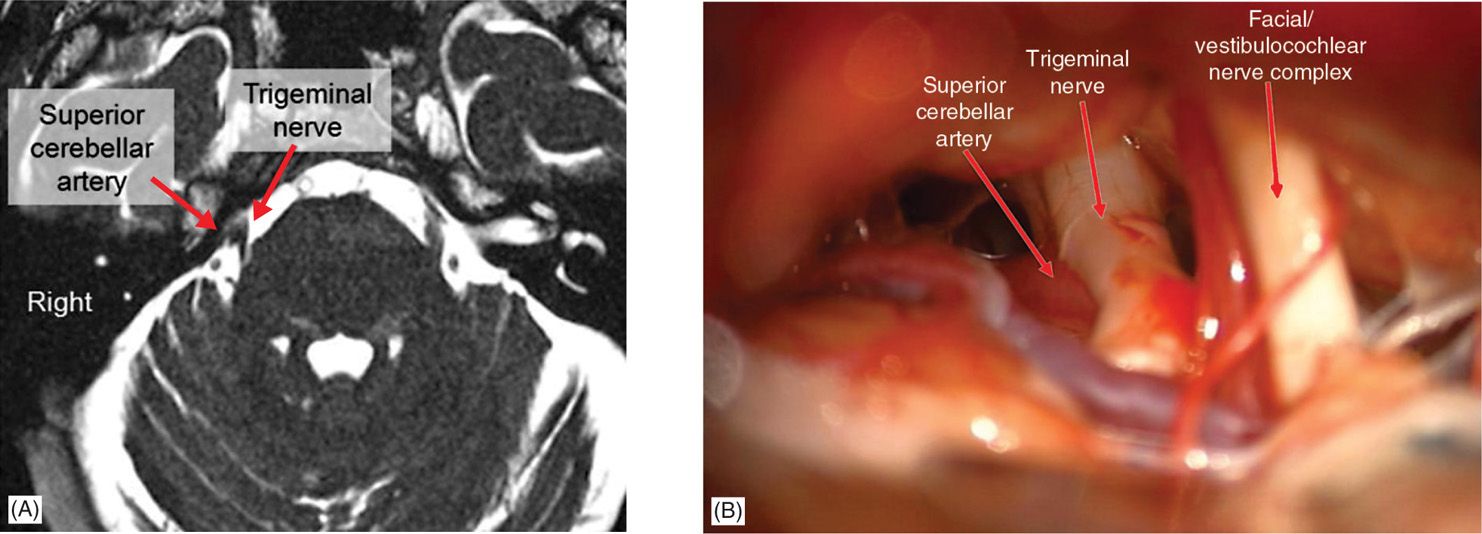
Figure 76-1. Arterial compression of trigeminal nerve. This is the most common cause of typical trigeminal neuralgia, usually as a result of compression by the superior cerebellar artery. (A) Axial FIESTA image demonstrates compression of the right trigeminal nerve midway between Meckel cave at the base of the skull and the entry zone into the brainstem (root entry zone). (B) Intraoperative image from the same patient demonstrates compression of the trigeminal nerve, with slight deformation of the nerve, by the superior cerebellar artery on the superior (left on the image) side of the nerve. Note the complex of the seventh (facial) and eighth (vestibulocochlear) nerves inferior to the trigeminal nerve (right on the image).
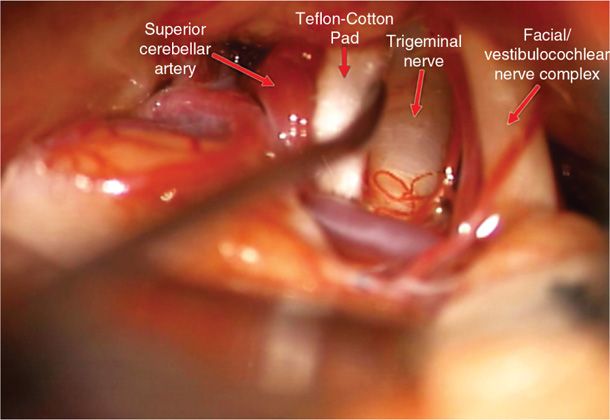
Figure 76-2. Microvascular decompression for trigeminal neuralgia. The superior cerebellar artery is mobilized away from the trigeminal nerve and a Teflon-coated cotton pad is placed between the artery and the nerve to maintain separation and cushion the nerve. Note the deformation of the nerve seen in Figure 76-1B is relieved by this maneuver.
• Care must be taken to ensure that the pad is sufficiently large and properly placed to minimize migration without being so large that it causes nerve injury.
• Any vascular structures touching the nerve at any point should be separated from the nerve, including arteries and veins, which can be either insulated from the nerve with a pad or coagulated and divided, depending upon anatomy and surgeon’s concerns regarding safety.
COMPLICATIONS
Several potential complications of surgery can occur and must be considered within the context of the efficacy profile prior to making a decision in favor of MVD.
• The most common adverse effects of surgery is hearing loss in the ipsilateral ear, which can occur in up to 10% of patients.
• The vestibulocochlear (eighth) nerve is adjacent to the trigeminal nerve, and often some dissection of the arachnoid over this nerve is necessary to permit adequate mobilization of the cerebellum and trigeminal nerve.
• The eighth nerve is very sensitive to injury, and retraction can nonetheless stretch the eighth nerve, leading to dysfunction.
• Vestibular dysfunction can occur as well.
• Inadvertent injury to the tenuous vascular supply for the eighth nerve can also be a cause of total hearing loss.
• Facial weakness due to injury to the facial (seventh) nerve can also occur, since this nerve runs in a complex with the eighth nerve at this level, but this is much less frequent due to the relative location of the seventh nerve and the greater resistance of that nerve to injury.
• Neurophysiological monitoring of the seventh and eighth nerves can be extremely helpful to understand if any dysfunction is occurring during surgery.
• Either partial or total sensory loss within one or more divisions of the trigeminal nerve can also occur following MVD, most likely due to damage to the nerve while placing the cotton pad.
• Cerebrospinal fluid (CSF) leak can also occur in most posterior fossa operations.
• Aseptic meningitis is often seen following MVD due to entry of blood into the subarachnoid space during the dissection.
• Aseptic meningitis can be minimized by limiting the amount of blood entering the subarachnoid space and through use of perioperative steroids.
PERCUTANEOUS LESIONING AT THE GASSERIAN GANGLION
For patients who are poor candidates for MVD based upon risk or age or for those who simply do not wish to undergo an open neurosurgical procedure due to risks or concern, percutaneous lesioning can be a very effective alternative. Also, for patients in pain crises who are not being controlled with more conservative therapies, the relative ease and rapid relief from these procedures can make them very useful in such circumstances. The goal of the RF procedure is to create analgesia without significant anesthesia.
• Percutaneous procedure involves placement of a needle through the foramen ovale, which is the point of exit of the mandibular (V3) division of the trigeminal nerve from the skull into the Gasserian ganglion composed of trigeminal nerve cell bodies. Usually this is performed in a radiology suite and fluoroscopy is used to help confirm proper location of the needle tip in the foramen ovale, and patients are briefly sedated for the procedure. Thin-cut computerized tomography (CT) precisely identifies the foramen ovale on reconstructed 3D models of the skull base. The image is then rotated to match the fluoroscopy image in order to help identify the foramen ovale based upon surrounding landmarks.
• Though single-plane fluoroscopy can be used, biplane fluoroscopy in an interventional neuroradiology suite is recommended to maintain the same submental and lateral views (Figure 76-3) throughout the procedure and to accelerate needle entry.
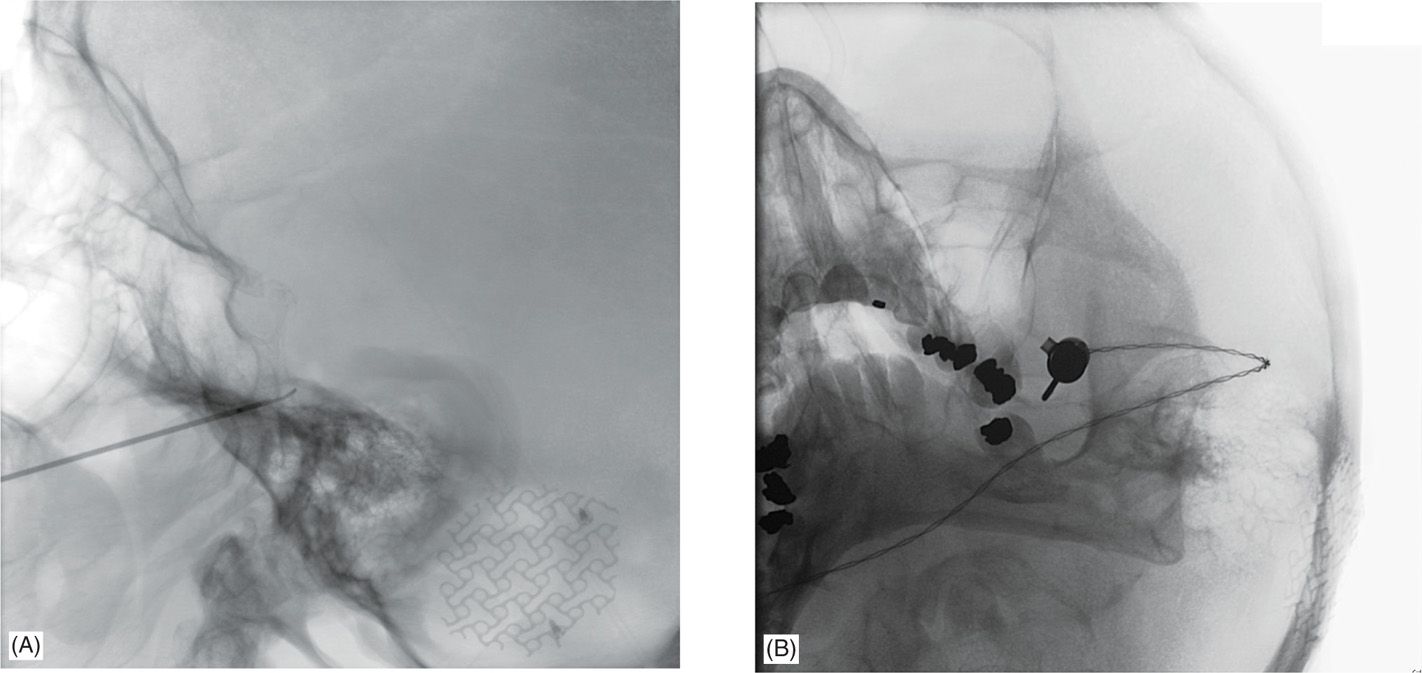
Figure 76-3. Approach for percutaneous radiofrequency lesion of the trigeminal nerve. Fluoroscopy in two planes is generally helpful to localize the foramen ovale and the proper location of the guide needle and lesioning probe. The V3 division of the trigeminal nerve passes through the foramen ovale, but the trajectory allows passage of the lesioning probe into the fibers of the Gasserian ganglion and thereby permits selective lesioning of any trigeminal division. (A) Lateral approach for a V2 lesion demonstrates the wider bore of the guide needle just before the clival line, with the lesioning probe extending roughly 4 mm out from the end of the guide needle and the tip extending just beyond the clival line. (B) Modified submental view demonstrates the guide needles with the attached lesioning probe passing through the center of the left foramen ovale. In this view, the needle appears to be more to the lateral edge of the foramen but the fluoroscopy arm was rotated in this instance to demonstrate the tip, which would be obscured by a straight view down the center.
• The ganglion is positioned in a straight line with V2 branch, 2 to 5 mm anterior and lateral to V3, and 3 to 5 mm medial past V1 at the level of the clival line (see Figure 76-3).
• Proper location of the needle is usually accompanied by egress of CSF upon entry into the subarachnoid space, although sometimes this is not clear and is less common in repeat procedures do to scarring from previous surgery.
• When the needle is properly placed, radiorequency (RF) thermocoagulation method is used to create a therapeutically effective lesion.
• An RF probe is passed through the central needle, with the tip positioned based upon the pattern of pain distribution and landmarks for the divisional nerve fibers outlined above.
• Patients are then generally awakened from sedation and a stimulating current (1 millisecond pulse width, 50-Hz frequency) is passed through the probe to functionally confirm proper location.
• If patients report sensing stimulation in a distribution is nonconcordant their pain pattern, then the probe can be slightly adjusted and retested.
• If patient has difficulty speaking or opening their mouth, this might suggest stimulation of the motor branch of the trigeminal nerve, and repositioning prior to lesioning should also be considered if this occurs.
• Patient is then resedated for an initial lesion at low temperature (60°C for 60 seconds).
• Patient should again be awakened and tested for sensory loss.
• Subsequent lesions at 90 seconds and progressively higher temperatures (usually in increments of 5°C) are performed, preferably with awake sensory testing in between lesions.
• After the first 2 to 3 lesions, patient can often undergo successive lesioning at the highest temperatures with less or no sedation due to the analgesia already created, which can facilitate active sensory testing during the higher-temperature lesions. Lesioning is usually discontinued when either a maximal temperature is reached (80°C) or when patients begin to report decreased sensation.
• Patients usually report worsening sensory loss 24 to 48 hours after the procedure, but this generally improves to leave patients with very limited or no sensory loss.
• Short-term pain relief following the procedure is generally excellent, with most reports indicating success rates of 70% to 80% to complete pain relief.
• The recurrence rate is reported as high as 50% at 5 years.
• Very low rates of CSF leak, infection, carotid artery injury due to misplaced needles, and corneal sensory loss have also been reported.
GLYCEROL INFUSION AND BALLOON COMPRESSION
As an alternative to RF lesioning, glycerol infusion or balloon compression may also be used. Percutaneous needle placement is similar to RF lesioning. For glycerol injection, some practitioners recommend cisternography to confirm proper placement prior to infusion, but others do not believe that this is necessary. Similarly, some reports recommend a small test infusion of glycerol to examine sensory changes which may reflect proper localization. For balloon compression, a balloon catheter is inserted through the needle passed through the foramen ovale and then inflated to compress the ganglion. Compression is usually performed for several minutes (roughly 5 minutes on average), with longer compression times associated with better therapeutic outcomes but also greater incidence of significant sensory loss. A major advantage of these approaches is the absence of need for repeated sedation followed by awake testing, since little or no sensory testing is required to complete these procedures. This usually makes these procedures much shorter than a properly performed RF lesion, and can also be very useful if patients are not very cooperative and have some cognitive impairment or a significant language barrier. Since they are less selective than RF, they can be associated with greater sensory loss in nerve divisions outside of the area of pain and have been reported to have somewhat greater incidences of V1 sensory loss and associated corneal denervation. Short-term pain relief is similar to RF, although some studies suggest that these are slightly lower than RF, and glycerol may be more preferable to balloon compression.
STEREOTACTIC RADIOSURGERY
Stereotactic radiosurgery (SRS), commonly referred as gamma knife surgery, is the least invasive of the accepted surgical therapies for TN. This is a specific method of SRS that has had the longest use and has been most widely studied. The procedure of SRS involves use of focused, high-intensity radiation to perform a noninvasive lesion of the trigeminal nerve. Since this is a consequence of the tissue response to radiation, the effect of SRS is often delayed from 1 to 3 months, so this may be less beneficial to patients in crisis with urgent need of pain relief. SRS can generally be performed safely on anticoagulated patients. Therefore, this may be a good option as well for patients who cannot safely stop anticoagulation for any length of time.
• The center of radiation is targeted to a point on the trigeminal nerve several millimeters distal to the brainstem and irradiate the brainstem at or less than the 20% isodose line.
• The goal is to radiate as close to the root entry zone of the nerve as possible while maintaining the radiation limit to the brainstem itself.
• For the gamma knife procedure, a rigid stereotactic frame is physically attached to the patient’s head using local anesthesia, followed by imaging (MRI or CT) and target planning various isodose lines around an isocenter target.
• In most cases, a single isocenter is used, but for difficult cases multiple isocenters can be used.
• Some recent technologies for SRS do not require placement of a frame, though the accuracy, safety, and efficacy of these devices for trigeminal neuralgia SRS have not been well established.
SRS using the gamma knife is generally very effective in the first 6 to 12 months following treatment, although it may have a slightly lower rate of patients who are pain free without medication. Most studies indicate that 40% to 70% of patients are pain free without medication between 6 and 12 months following the procedure, although generally 70% to 80% of patients report satisfactory pain relief at 1 year regardless of whether or not they have discontinued medication. As with percutaneous procedures, a substantial rate of recurrence is seen progressively over the years. Some studies have reported success with repeat procedures in patients with long-term recurrences. The major complication of SRS is persistent decrease or loss of facial sensation in one or more distributions of the trigeminal nerve in 10% of patients to be as high as 30% in some studies. Generally 70 to 80 Gy is delivered to the isocenter near the root entry zone to achieve good outcomes, with lower doses of radiation associated with poorer outcomes while higher doses are associated with greater sensory loss but without clear additional therapeutic benefit. The patients who fail percutaneous lesioning or MVD are unlikely to benefit from SRS and may in fact have an alternative diagnosis to classic TN.
NEUROMODULATION THERAPIES
Neurostimulation has long been used to treat chronic pain. Currently, spinal cord stimulation and certain types of peripheral nerve stimulation are FDA-approved for pain in the arms, legs, and back. While neurostimulation for facial pain is increasingly being explored, it is currently considered an off-label use of these devices. A potential advantage of neurostimulation is that it is nondestructive and is reversible procedure, which can be effective for difficult cases with unclear diagnosis or where multiple prior therapies have failed. Neurostimulation for pain involves placement of an externalized lead for several days to a week as trial followed by implantation of a permanent system. There have been two primary types of neurostimulation used for facial pain: motor cortex stimulation and peripheral facial stimulation.
MOTOR CORTEX STIMULATION
Motor cortex stimulation (MCS) is an intracranial procedure where a stimulating electrode is placed on the surface of the brain in order to stimulate the motor cortex. The mechanism of action remains unclear, but it is believed that MCS may stimulate U-fibers which connect to sensory areas. MCS has been used to treat a variety of complicated pain syndromes like:
• Phantom-limb pain
• Central pain syndromes
• Poststroke pain syndrome
• Intractable trigeminal neuropathic pain
• Patients who failed SCS or peripheral nerve stimulation
TN category includes a far broader group of patients than simply classic TN, and therefore it remains unclear if results of treatment specifically in TN would be any better or worse than in the broader group as a whole. Most practitioners used approved spinal cord stimulation paddle electrodes to place on the brain, generally outside the dura to avoid intradural hemorrhage or injury. Due to the invasive nature of this craniotomy procedure, test stimulation is usually performed by attaching the end of the paddle lead to a second lead extension, which is then externalized from a separate stab wound behind the craniotomy incision line. After 1-week trial, if patients report adequate pain relief, then the externalized extension is removed and the lead is attached to a new lead extension which is tunneled to a pulse generator, usually placed in the anterior chest wall.
Most studies have defined a good response as greater than 40% to 50% pain relief in 65% to 75% of patients with trigeminal neuropathic facial pain at 1 year. Complications of surgery are infection, bleeding, and hardware-related complications in 5% to 10% of patients. Ten percent of patients reported to have perioperative seizures, and that may be due to electrical stimulation of the cerebral cortex which is more likely to lead to seizures than subcortical stimulation. However, these appear to be isolated seizures, as long-term epilepsy has not been reported in these patients. As the permanent complications appear to be rare, it may be a reasonable consideration as an off-label treatment for patients who have not responded well to conservative therapy and are not good candidates for more traditional TN surgery.
PERIPHERAL FACIAL STIMULATION
As with MCS, peripheral facial stimulation (PFS) is a nonapproved application of neurostimulator system for certain peripheral conditions like:
• Difficult cases of post-traumatic or other forms of trigeminal neuropathic pain
• Patients with refractory atypical facial pain
• TN symptoms unresponsive to other surgical therapies
Due to relatively low morbidity of peripheral stimulation procedure, the ability to trial stimulation prior to permanent implantation and the reversibility of this procedure favor consideration of this technique for select appropriate patients. In this case, a thin lead used for percutaneous stimulation is placed in the subcutaneous tissue of the face in order to stimulate branches of the supraorbital (V1) or infraorbital (V2) sensory nerves. Mandibular stimulation can also be performed, although the extreme mobility of the mandible on an almost continuous basis (talking, eating) raises concerns regarding lead erosions or fractures compared with less mobile sites.
• Patients are generally sedated for these procedures, although general anesthesia can be more useful as proper placement can be somewhat painful.
• As awake test stimulation is less critical for proper localization than for spinal stimulation, general anesthesia can actually facilitate better placement without patient discomfort.
• Fluoroscopy is helpful to confirm that leads are placed in good position and, in particular, to confirm that the tip of the lead is just lateral to midline.
• The lead should be placed just above V1 or below V2 the orbital rim in order to stimulate the nerves just as they exit their respective foramina (Figure 76-4).
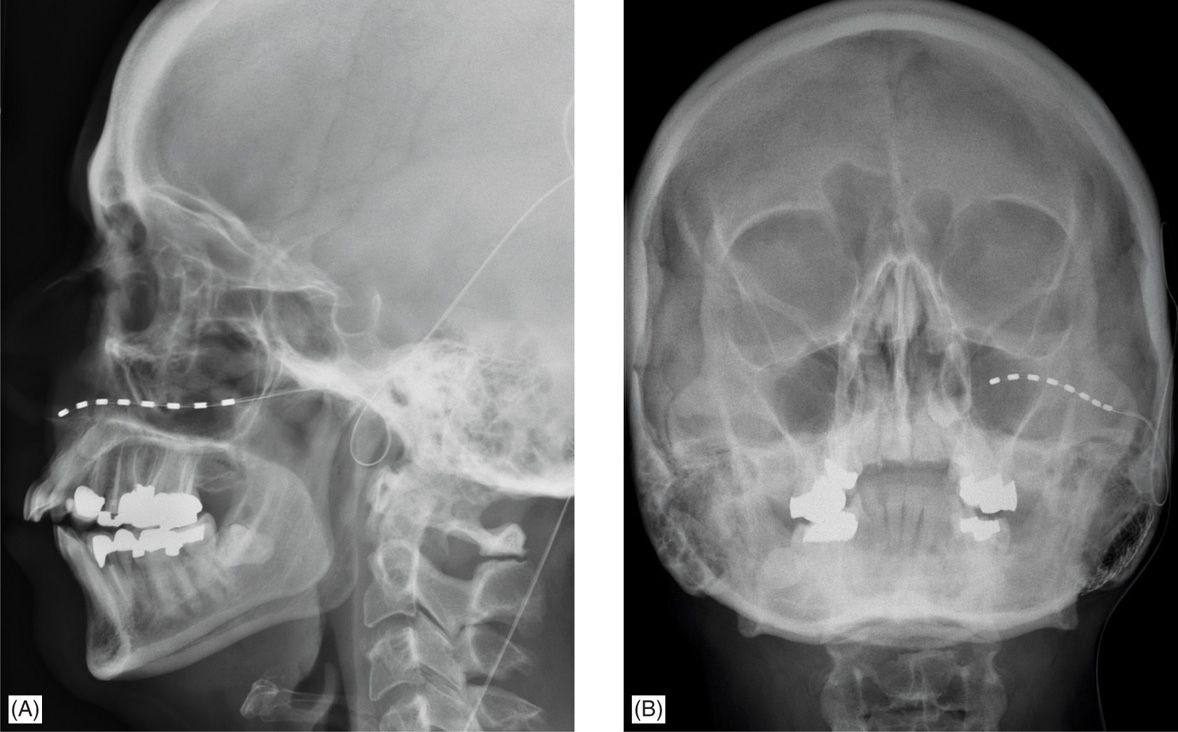
Figure 76-4. Localization of stimulating electrode for peripheral facial stimulation of the trigeminal nerve. In this case, the stimulation was for pain in the V2 distribution and therefore the electrode was placed to stimulate the infraorbital nerve. (A) Lateral skull x-ray demonstrates the infraorbital location of the percutaneous stimulating electrode (B) AP skull x-ray from the same patient again demonstrates the infraorbital placement of the stimulating electrode. The tip of the electrode usually abuts the lateral edge of the nasal bones.
• A percutaneous needle is bent slightly to create a curve, with a stab incision for an entry site made just behind the hairline.
• For a trial lead, a second stab is made in a more posterior position, and the same percutaneous needle is used as a tunneler to tunnel the excess lead from the entry site to the more posterior site.
• The lead is anchored with a suture and test stimulation is performed for 1 week. After a successful trial, a similar technique is used to implant a new permanent lead and this is then tunneled to a pulse generator placed in the anterior chest wall.
• A loop of the lead should be left at the entry site for strain relief from movement of the neck.
The most widespread use of peripheral stimulation in the head and neck has been stimulation of the occipital nerves for occipital neuralgia and migraine headaches. Some studies have reported trigeminal peripheral stimulation for trigeminal neuropathic pain and postherpetic neuralgia. The studies reported 70% of patients to have good response to trial stimulation and go on to permanent implantation. Of those receiving permanent implants, 50% to 75% of patients maintain greater than 50% pain relief at 2 years. Currently this remains an off-label indication for these devices, and more studies with longer-term follow-ups are needed.
SUMMARY
Facial pain can be one of the more difficult and refractory conditions to manage for pain specialists, neurologists, and neurosurgeons. Medical therapy is clearly the first choice and can be effective in many cases. However, frequently patients do not adequately respond to trials of multiple medications or they become refractory to treatment. In these situations, a variety of surgical therapies can cure or dramatically relieve pain. A proper initial diagnosis is essential, since most accepted surgical therapies are primarily useful for true, idiopathic trigeminal neuralgia. However, advances in neuromodulation now provides a variety of novel options for patients who either develop recurrence of pain following more traditional surgery or for those who do not have classical trigeminal neuralgia. At present, these approaches use off-label applications of devices approved for other indications, which can somewhat limit utility of these methods. Nonetheless, these can be considered in isolated cases and further studies should help increase both our understanding of the role of neuromodulation for refractory pain and the eventual broad acceptance of this option.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






