Fig. 20.1
(a) Suprascapular nerve and its branches of the left shoulder. Superior articular branch (Br.SA) supplies the coracohumeral ligament, subacromial bursa, and posterior aspect of the acromioclavicular joint capsule. Inferior articular branch (Br.IA) supplies the posterior joint capsule. Ac indicates acromion; Br.IS branch to the infraspinatus muscle, Br.SS branch to the supraspinatus muscle, CP coracoid process, SS scapula, spine, TSL transverse scapula ligament (Reproduced with permission from Philip Peng Educational Series). (b) Posterior view of the shoulder. (1) Supraspinatus muscle, (2) spine of the scapula, (3) deltoid muscle, (4) suprascapular artery, (5) suprascapular nerve, (6) teres minor muscle, (7) infraspinatus muscle, (8) teres major muscle, (9) latissimus dorsi muscle (with permission from Danilo Jankovic)
Shortly after passing through the suprascapular notch, the SSN emits two branches: one is the motor nerve for the supraspinatus muscle [16–18] and the other is known as the superior articular branch. The latter nerve is sensory and supplies the coracoclavicular, coracohumeral ligaments, the acromioclavicular joint, the glenohumeral joint (posterior and superior aspects), and the subacromial bursa [12, 19, 20]. The main trunk then exits the suprascapular fossa by curving around the lateral border of the scapula spine through a fibro-osseous tunnel formed by the spinoglenoid ligament and the spine of the scapula and terminates in motor branches to the infraspinatus muscle [18, 20] (Fig. 20.2).
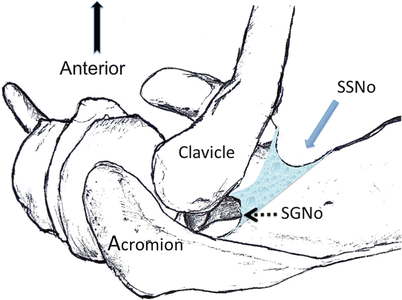

Fig. 20.2
Superior view of the left shoulder. The course of the suprascapular nerve (shaded) enters the suprascapular fossa through the suprascapular notch (SSNo) and then enters the infrascapular fossa through the spinoglenoid notch (SGNo). The clavicle (Cl) and coracoid process (Co P) are also displayed in the diagram (Reproduced with permission from Philip Peng Education Series)
The anatomy of the suprascapular notch is important for several reasons. The nerve is susceptible to injury and impingement at the level of the notch as it passes beneath the STSL [21, 22]. This site represents an attractive region for SSN blockade as the nerve has not divided yet. The variable shape of the notch has been described and has been categorized into different types [23, 24] (Fig. 20.3). In the adult, the most common type is a U-shaped or semicircular notch (Type 1 and 2 in Fig. 20.3) [23]. The notch is absent or converted into a foramen by the ossified STSL in 15 % of the specimens [24].
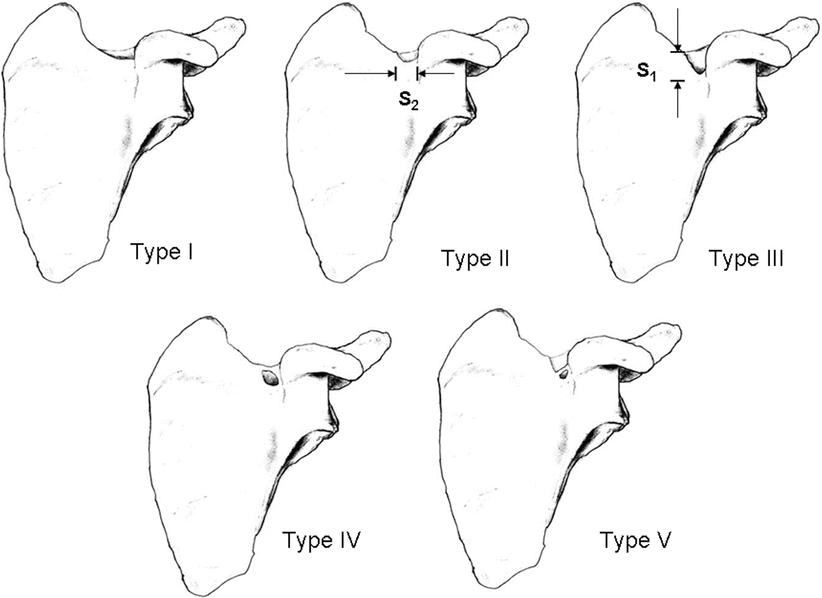

Fig. 20.3
Variation of morphology of the suprascapular notch. Type I indicates no notch (8.3 %); type II, notch with greater transverse diameter, S2 (41.85 %); type III, notch with greater vertical diameter, S1 (41.85 %); type IV, bony foramen (7.3 %); type V, notch with bony foramen (0.7 %) (Adapted from Natsis et al. [24]; Reproduced with permission from Philip Peng Education Series)
Technique of Localizing the Suprascapular Nerve
Traditionally, suprascapular nerve blockade has been performed via use of anatomical landmarks (blind techniques) [6]. More recently, the use of imaging guidance to more accurately guide needle placement has been described [6].
Blind Techniques
Various landmark approaches have been described and can be grouped into posterior, superior, and lateral approaches. The posterior approach attempts to block the SSN at the level of the suprascapular notch [25–29], while the superior approach aims to block the SSN by surrounding the nerve with local anesthetic on the floor of the supraspinous fossa [30, 31]. A lateral approach to localize the suprascapular nerve has also been described [32, 33]. To improve accuracy, the SSN has been localized using nerve stimulation [34, 35], paresthesia [36], and EMG [37]. The popularity of these techniques has reduced with the introduction of radiological guidance which will be discussed in the next section.
Posterior Approach
The posterior approach is generally performed while the patient sits on the operating table with the ipsilateral arm lying by his or her side [25, 27, 38]. To minimize the risk of pneumothorax, the scapula can be elevated from the posterior chest wall by repositioning the ipsilateral hand to the opposite shoulder, thereby increasing the potential distance the needle must travel from the skin to chest wall [36]. A 22-gauge, 3-in. needle is generally used.
The superficial landmarks described in the posterior approach techniques serve to guide the needle to slide into the notch. As discussed in the anatomy section (above), the notch is not a defined structure in 15 % of the population. Furthermore, the potential complication of this approach is pneumothorax as the trajectory of the needle is toward the thoracic cavity.
Superior Approach
The superior approach [30, 31] was initially described to permit SSNB performed in patients in the supine position but the sitting position is the preferred position in clinical practice.
The needle entry point for the superior approach has been described as cephalad and lateral to the midpoint of the spine of the scapula [30]. In general, the needle is directed to the lateral half of the floor of the suprascapular fossa because the supraspinatus muscle is attached to the medial half. A 1.5-in. needle is usually sufficient to reach the floor of the scapular fossa. Potential advantages of this approach include ease of access, no reference to the notch, and extremely low risk of pneumothorax [30, 31].
Lateral Approach
In this approach, the needle entry point is at a landmark known as the “Neviaser portal” [32, 33, 36]. The site can be found by palpating the “soft spot” 1 cm medial to the junction of the clavicle and the scapular spine (Fig. 20.4). The needle is inserted and angled toward the coracoid. When bone contact is made (scapula), the needle is then directed anteriorly until no bone is felt, then retracted marginally, and moved posteriorly until bone again is felt [32]. This positions the needle tip at the coracoid base of the supraspinous fossa [32]. As this places the needle tip in the supraspinous fossa, an adequate block is achieved by flooding the plane with local anesthetic. Initial reports advised using 25 mL of local anesthetic solution with this lateral approach [32]. Subsequently, a modified lateral SSNB approach was published which demonstrated sufficient spread of solution throughout the supraspinous fossa with a volume of 5 mL (see below) [33].
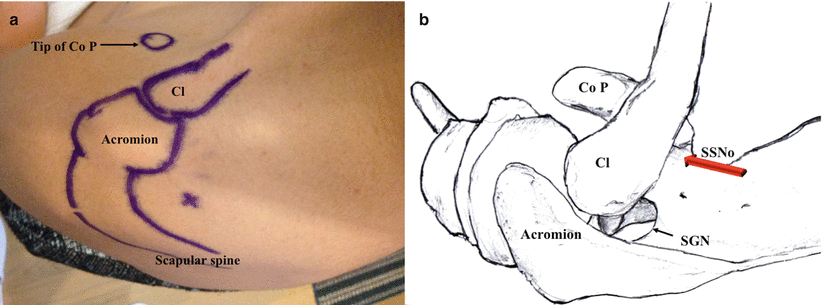

Fig. 20.4
(a) Superficial anatomy demonstrating landmarks for the lateral approach to SSNB. The x marks the site known as Neviaser portal. The o represents the coracoid process. (b) Final location of the needle at the base of the coracoid in the supraspinatus fossa where the SSN curves around the coracoid and heads to the glenohumeral joint (red arrow). Injection of high-volume local anesthetic floods the area where the SSN lies (Reproduced with permission from Philip Peng Education Series)
Comparison of Blind Approaches
Despite the many number of approaches and techniques published to date, few studies have actually compared them. An old study on pulsed radiofrequency lesioning of the SSN [39] compared 4 commonly used blind techniques [25, 26, 29, 40], in terms of the final position of the needle tip relative to the suprascapular notch with radiographical correlation. They found that the needle tip was usually a significant distance from the notch such that a heat lesion would not affect the SSN in all techniques. When comparing the blind methods, they found that the approach suggested by Granirer [29] offered the best approximation of “needle tip to notch” [39].
Image-Guided Techniques for SSNB
Techniques using imaging guidance such as fluoroscopy [41], computed tomography (CT) [34], and more recently ultrasound [7, 8, 35, 37, 42] have been described.
Conventional Imaging
Fluoroscopy and CT have been described to locate the suprascapular notch [34, 41]. For the fluoroscopic technique, the patient is placed in the prone position. A C-arm is then used to identify the notch (Fig. 20.5) [41]. The suprascapular notch will be seen superior to the spine of the scapula, medial to the coracoid process, and lateral to the rib margins (Fig. 20.5) [41]. To obtain an optimal image, the C-arm will often need to be obliquely angled away from the side of the proposed block and in the cephalocaudad orientation [41]. Visualization of the suprascapular notch is further improved when the ipsilateral arm is placed above the shoulder (“arm up” position) compared to when the affected arm is placed by the side (“arm down position”) (91 % vs. 47 %; p < 0.0001) [43].
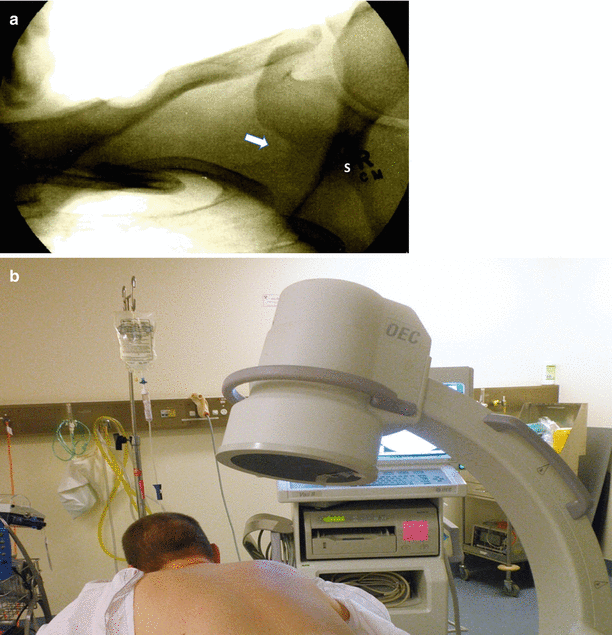

Fig. 20.5
(a) Radiograph of the right suprascapular notch. S indicates spine of scapula. White arrow points to the suprascapular notch. (b) C-arm positioning for imaging the suprascapular notch. The patient is placed in prone position. The C-arm is positioned over the shoulder. To image the suprascapular notch, the C-arm is rotated oblique to the treated side and angled cephalocaudal (Reproduced with permission from Philip Peng Education Series)
Ultrasound-Guided Suprascapular Nerve Blockade
Ultrasound (US) guidance is the most recent imaging technique that has been employed to assist SSNB. Several articles have been published describing this technique [7, 8, 35, 37, 42]. Ultrasound guidance is a relatively new technique, and various methods utilizing sonography have been published [7, 8, 35, 37, 42]. Both anterior and posterior approaches have been described. The anterior approach targets the suprascapular nerve shortly after it branches from the brachial plexus (in the supraclavicular area) underneath the omohyoid muscle [44]. The major limitation of this approach is the proximity to the brachial plexus (median 8 mm; range 4–15 mm). For the purpose of this chapter, the more widely practiced technique of US-guided SSNB will be described.
The ideal site to perform SSNB with ultrasound is at the floor of the suprascapular fossa, between the suprascapular notch and spinoglenoid notch [8] (Fig. 20.2). At this site, the SSN runs along the floor of the suprascapular fossa covered by the fascia of supraspinatus in a natural compartment, which will contain the spread of the local anesthetic or injectate [8]. Applying an ultrasound-guided injection technique approximated the needle tip to the nerve and has been shown to achieve a complete block with a reduced volume of local anesthetic [45]. A small volume (5 mL) of injectate will result in adequate flooding of the nerve [46] with minimal spread to the brachial plexus [33]. Furthermore, this target is independent of the suprascapular notch, which can be absent in some individuals. The risk of pneumothorax is substantially reduced because of the direction of the needle [47].
The ultrasound-guided SSNB may be performed with the patient either in the sitting or prone position. A high-frequency (7–13 Hz) linear ultrasound probe is used. The probe is placed in a coronal plane over the suprascapular fossa (Fig. 20.6). The probe should be placed such that the midpoint of the probe divides a line between the coracoid process and posterior aspect of the acromion (Fig. 20.6). A slight anterior tilt is placed on the probe to improve visualization of the target structures (Fig. 20.6). In this view, the identifiable structures (from superficial to deep) are the trapezius muscle, the supraspinatus muscle, and the suprascapular fossa floor (Fig. 20.6). The suprascapular nerve and artery are commonly seen on the floor running underneath the supraspinatus muscle layer (Fig. 20.6).
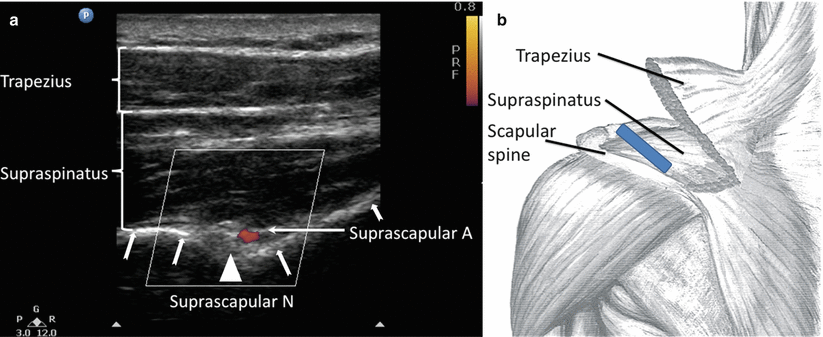

Fig. 20.6
(a) Ultrasonographic image of the suprascapular nerve on the floor of the scapular spine between suprascapular notch and spinoglenoid notch. Both suprascapular nerve and artery run underneath the fascia of supraspinatus muscle. Suprascapular A and N indicate suprascapular artery and nerve. Bold arrows outline the floor of the scapula fossa. (b) Approximate position of the ultrasound probe (dark rectangle). The patient can be in sitting or in prone position. Ultrasound scanning is performed with a linear ultrasound probe (7–13 MHz) placed in a coronal plane over the suprascapular fossa with a slight anterior tilt. The probe is placed in an orientation such that it is in the short axis to the line joining coracoid process and acromion (reflecting the position of the spinoglenoid notch). The trapezius muscle was removed to show the underlying supraspinatus muscle (Reproduced with permission from Philip Peng Education Series)
SSNB is performed by passing a 22-gauge, 3-in. needle in an in-plane approach from medial to lateral.
Imaging to Improve Needle Localization of the Suprascapular Nerve
One randomized single-blind trial compared the blind approach to SSNB with a CT-guided approach [48]. This study did not find any significant difference between the blind or CT-guided SSNB in terms of pain and disability scores [48]. Both groups showed significant improvement post SSNB [48]. There were no significant adverse effects in either group, and patient satisfaction scores were high [48].
The efficacy of ultrasound-guided SSNB was compared to the landmark-based technique [9]. In this study, patients with chronic nonspecific shoulder pain were randomized with 25 patients in each group. The investigators found that initially both groups improved in terms of pain relief. However, the analgesic effect was better sustained at 1 month in the ultrasound-guided group compared to the control group [9]. Furthermore, while there were no complications in the ultrasound group, the control group recorded 2 cases of arterial puncture and 3 cases of direct nerve injury with neurological deficit [9].
In summary, various approaches have been described for the blockade of SSN. Disadvantages of the approach using the notch as a landmark are the potential absence of the notch in some individuals and the potential risk of pneumothorax. The superior approach may negate these disadvantages. On limited evidence, these studies would suggest ultrasound is useful in approximating the block needle in the vicinity of the SSN and thereby increasing efficacy and reducing complications of SSNB. The majority of recent studies utilizing US-guided SSNB for acute and chronic shoulder pain management may reflect this.
Substances Used for Blockade of the Suprascapular Nerve
When the needle is placed in proximity to the SSN, several methods of nerve blockade have been published. The commonly used methods include local anesthetic, steroids, pulsed radiofrequency, and chemical neurolysis. These may be used alone or in combination. Bupivacaine (0.25 or 0.5 %) with epinephrine (1:200,000) is the popular local anesthetic agent [30, 31, 49–53]. Injectate volume is highly variable in the literature. However, some authors argue 5 mL is the optimal volume based on morphological evidence [46].
For treatment of chronic shoulder pain, injectable steroid (methylprednisolone) usually is added to the local anesthetic solution [5, 9, 54–56]. However, the value of this practice has been questioned by a double-blind study [52] demonstrating that the addition of methylprednisolone fails to confer any benefit.
SSNB achieved with radiofrequency or cryolesion provides a long-lasting effect that can endure for up to 18 months [11, 39, 57–59]. Furthermore one of these studies demonstrated a significant reduction in pain, improvement in function, and a reduction in analgesic medication (81 % of study patients) following pulsed RF of the SSN [11].
The use of chemical neurolysis for SSNB has mainly been in the form of case reports [39, 60]. Injection of phenol causes protein coagulation and necrosis when applied directly to the nerve, thereby alleviating pain. A larger study involved 16 patients with shoulder pain secondary to rheumatoid arthritis. These patients received SSNB with prilocaine (4 mL) and 6 % aqueous phenol (4 mL) with significant reduction in pain and improved shoulder range of motion at 13-week follow-up [61].
Complications
SSNB is a safe procedure with a generally low rate of complications (Table 20.1).
Table 20.1
Complications of suprascapular nerve block and rhizotomy
Study authors | Type of study | No. of patients | Clinical indication for SSNB or SSN rhizotomy | Complications reported (numbers) |
|---|---|---|---|---|
Shanahan et al. (2012) [62] | O (multicenter study 2003–2009) | 1,005 | Chronic shoulder pain | 6 side effects: Transient dizziness (3) Transient arm weakness (2) Facial flushing (1) |
aEyigor et al. (2010) [57] | R | 50 | Chronic shoulder pain | No complications reported |
Gorthi et al. (2010) [9] | R, C | 50 | Chronic shoulder pain | US group—no complications; blind technique, arterial puncture (2), direct nerve injury (1) |
Martinez-Barenys et al. (2010) [63] | R | 74 | Ipsilateral shoulder pain post-thoracotomy | No complications reported |
Saha et al. (2010) [64] | O | 178 | Ipsilateral shoulder pain post-thoracotomy | No complications reported |
Mitra et al. (2009) [65] | O | 28 | Adhesive capsulitis | No complications reported |
aLiliang et al. (2009) [11] | O | 11 | Chronic shoulder pain | Puncture wound pain for 1 week (1) |
aKane et al. (2008) [10] | O | 12 | Painful cuff tear arthropathy in patients unfit for surgery | No complications reported |
Checucci et al. (2008) [4] | O | 20 | Patients undergoing arthroscopic procedures for rotator cuff disease | No complications reported |
Jerosch et al. (2008) [66] | R | 260 | Arthroscopic and shoulder surgery | No complications reported |
Price (2007) [67] | O | 40 | Arthroscopic and open shoulder surgery | No complications reported from SSNB |
Di Lorenzo et al. (2006) [47] | R | 40 | Rotator cuff tendinitis | No major complications |
Taskaynata et al. (2005) [56] | R | 60 | Chronic shoulder pain | No complications reported |
Singelyn et al. (2004) [68] | R, C | 120 | Arthroscopic shoulder surgery | No complications reported |
Shanahan et al. (2004) [48] | R, SB | 67 | Degenerative joint or rotator cuff disease | No complications reported |
Schneider-Kolsky et al. (2004) [34] | O | 40 | Chronic shoulder pain | No complications reported |
Neal et al. (2003) [69] | R, DB, C | 50 | Acromioplasty, rotator cuff repair, or combination of both | No complications reported |
Shanahan et al. (2003) [5] | R, DB, C | 83 | Shoulder pain from rheumatoid arthritis and/or degenerative disease of the shoulder | Minor bruising (1) |
Tan et al. (2002) [70] | R, DB, C | 44 | Ipsilateral shoulder pain post-thoracotomy | No complications reported |
Karatas and Meray (2002) [71] | R, SB | 41 | Adhesive capsulitis | No complications reported |
Dahan et al. (2000) [72] | R, DB, C | 34 | Frozen shoulder | No major complications reported |
Jones and Chattopadhyay (1999) [73] | R | 30 | Frozen shoulder | No major complications reported |
Lewis (1999) [61] | O | 16 | Rheumatoid or osteoarthritis of shoulder | No complications reported |
Ritchie et al. (1997) [3] | R, DB, C | 50 | Arthroscopic shoulder surgery | No complications |
Dangoisse et al. (1994) [31] | O | 12 | Frozen shoulder (6 patients), others (6 patients) | Sensation of heaviness in the arm (1), numbness and aching shoulder (1) |
Gado and Emery (1993) [52] | R, DB | 26 | Rheumatoid arthritis | No complications reported |
Vecchio et al. (1993) [74] | R, C | 28 | Rotator cuff tendinitis | Mild aching in the injection area (16) |
Wassef, (1992) [49] | O | 9 | Frozen shoulder | No complications reported |
Emery et al. (1989) [51] | R, DB, C | 17 | Rheumatoid arthritis | No complications reported |
aBrown et al. (1988) [39] | O | 22 | Rheumatoid arthritis | Impaired abduction (1) |
The largest study to date retrospectively analyzed 1,005 SSNBs performed by multiple clinicians in multiple centers over a 6-year period [62]. There were no major complications reported [62]. There were only 6 minor adverse events which included transient dizziness (n = 3), transient arm weakness (n = 2), and facial flushing (n = 1) [62].
The possible complications are pneumothorax (<1 %), intravascular injection, residual motor weakness, local trauma, and vasovagal response of the patient.
Suprascapular Nerve Blockade in Clinical Practice
Acute Pain
The studies investigating the efficacy of SSNB in acute pain states are summarized in Table 20.2.
Table 20.2
Suprascapular nerve block for acute pain control
Study authors | Type of acute pain | Study design | Number of participants | Results | Conclusions |
|---|---|---|---|---|---|
SSNB as the only form of regional anesthesia | |||||
Jerosch et al. (2008) [66] | Arthroscopic shoulder surgery: mixed | Prospective, non-randomized study. Comparison of 2 consecutive cohorts | 260 patients Received SSNB = 130 No nerve block = 130 | No difference in baseline VAS scores. Postoperatively, significant reduction in VAS scores at 24, 48, and 72 h in the SSNB group. No complications of SSNB | SSNB is effective in reducing postoperative shoulder pain in arthroscopic shoulder surgery. SSNB is associated with minimal complications |
Singelyn et al. (2004) [68] | Arthroscopic shoulder acromioplasty | Prospective, randomized, blind study | 120 patients randomized to 4 treatment groups: SSNB = 30 IALA = 30 ISBPB = 30 Control (no regional analgesia) = 30 | No significant difference in pain scores between control and IALA groups. SSNB and ISBPB reported significantly less pain than the other 2 groups. The ISBPB group had significantly less pain on movement than the SSNB group. Only the ISBPB group recorded significantly less morphine consumption and higher satisfaction | ISBPB is the most efficient regional technique for arthroscopic shoulder acromioplasty. SSNB improves analgesia for arthroscopic acromioplasty but is less efficient than ISBPB. When ISBPB is contraindicated, SSNB is a clinically appropriate alternative |
Ritchie et al. (1997) [3] | Arthroscopic shoulder surgery | Randomized, double-blind, placebo-controlled study | 50 patients randomized to: Placebo = 25 SSNB = 25 | VAS significantly lower in SSNB group at 120, 180 min. VPS score significantly lower in SSNB group at 120, 180, and 240 min. Significantly reduced morphine consumption (SSNB group) on the day of surgery. Significantly less nausea and vomiting in SSNB group. Reduced stay in ambulatory surgical unit | SSNB is an effective regional anesthetic technique for arthroscopic shoulder surgery in terms of improved analgesia, reduced opioid requirements, and less nausea and vomiting |
Martinez-Barenys et al. (2010) [63] | Ipsilateral post-thoracotomy shoulder pain | Randomized, single-blind study | 74 patients 1st group: phrenic group (PNI) received 10 mL 2 % lidocaine into perinephric fat pad prior to closure = 37 2nd group: SSNB with 10 mL 0.5 % bupivacaine at completion of surgery = 37 | Shoulder pain intensity was significantly lower in the PNI group compared with the SSNB group | Shoulder pain post-thoracotomy does not appear to arise from the shoulder joint. This study suggests that pain arises from diaphragmatic irritation. Therefore, routine preemptive blockade of the suprascapular nerve is not recommended |
Saha et al. (2010) [64] | Ipsilateral post-thoracotomy shoulder pain | Retrospective case review of post-thoracotomy patients | 178 patients post-thoracotomy. New-onset shoulder pain post-thoracotomy = 92 (51 %). 34 patients (27 %) with localizing signs suggestive of musculoskeletal origin underwent SSNB | 29 of 34 patients reported satisfactory pain relief following SSNB | In patients with post-thoracotomy shoulder pain and who have localizing signs suggestive of musculoskeletal origin, SSNB is an effective treatment. However, SSNB is not the treatment per se for post-thoracotomy shoulder pain as the musculoskeletal system is responsible for less than one third of cases |
Tan et al. (2002) [70] | Ipsilateral post-thoracotomy shoulder pain | Double-blind, randomized, placebo-controlled study | 44 patients who had undergone thoracotomy under general anesthesia and mid-thoracic epidural. 30 patients experienced shoulder pain within 2 h postsurgery and were randomized to: SSNB with 10 mL 0.5 % bupivacaine = 15 Control: SSNB with 10 mL 0.9 % saline = 15 | No significant decrease in VAS or VRS in patients receiving SSNB with bupivacaine | SSNB not effective for ipsilateral shoulder pain post-thoracotomy |
SSNB in combination with another regional technique | |||||
Lee et al. (2014) [75] | Ambulatory arthroscopic shoulder surgery | Prospective randomized controlled trial | 42 patients 21 patients received US-guided SSNB and axillary nerve block 21 patients received US-guided SSNB alone | Addition of US-guided axillary nerve block to SSNB resulted in reduced pain scores (VAS) in the first 24 h postsurgery, compared to SSNB alone | Axillary nerve block improves analgesic efficacy of SSNB for ambulatory arthroscopic shoulder surgery |
Checucci et al. (2008) [4] | Arthroscopic shoulder surgery | Case series | 20 consecutive patients: each patient received a SSNB and an axillary nerve block as the sole anesthetic for the operation, with midazolam sedation | All patients were able to have surgery under the combination block. No patients required opioids, analgesics, or general anesthesia. Postoperative pain control was effective with negligible use of non-opiate analgesics. No opiate analgesic was required postoperatively | SSNB in combination with axillary nerve block is sufficient for arthroscopic shoulder surgery |
Price (2007) [67] | Shoulder surgery: arthroscopic and open. Postoperative analgesia in patients who had ISBPB failure | Retrospective case series | 40 patients with ISBPB failure received combined SSNB and axillary nerve block | 57 % of cases required no morphine in PACU. 83 % of cases required no morphine overnight. Complications: radial nerve blockade which resolved (2/70 cases) | If ISBPB fails, combined SSNB and axillary nerve block is effective in providing postoperative analgesia for shoulder surgery |
Neal et al. (2003) [69] | Ambulatory nonarthroscopic shoulder surgery | Prospective randomized study | 50 patients SSNB and ISBPB: general anesthesia = 25 Sham injection and ISBPB: general anesthesia = 25 | Addition of SSNB significantly delayed the time to first significant report of pain. However, addition of SSNB did not improve PACU measures, 24-h assessment of pain, supplemental analgesic use, or QOL measures | SSNB combined with ISBPB does not significantly improve outcomes in ambulatory nonarthroscopic shoulder surgery |









