Key Concepts
 Neuraxial anesthesia greatly expands the anesthesiologists’ armamentarium, providing alternatives to general anesthesia when appropriate. Neuraxial anesthesia may also be used simultaneously with general anesthesia or afterward for postoperative analgesia. Neuraxial blocks can be performed as a single injection or with a catheter to allow intermittent boluses or continuous infusions.
Neuraxial anesthesia greatly expands the anesthesiologists’ armamentarium, providing alternatives to general anesthesia when appropriate. Neuraxial anesthesia may also be used simultaneously with general anesthesia or afterward for postoperative analgesia. Neuraxial blocks can be performed as a single injection or with a catheter to allow intermittent boluses or continuous infusions.
 Performing a lumbar (subarachnoid) spinal puncture below L1 in an adult (L3 in a child) usually avoids potential needle trauma to the cord.
Performing a lumbar (subarachnoid) spinal puncture below L1 in an adult (L3 in a child) usually avoids potential needle trauma to the cord.
 The mechanisms of spinal and epidural anesthesia remain speculative. The principal site of action for neuraxial blockade is believed to be the nerve root.
The mechanisms of spinal and epidural anesthesia remain speculative. The principal site of action for neuraxial blockade is believed to be the nerve root.
 Differential blockade typically results in sympathetic blockade (judged by temperature sensitivity) that may be two segments or more cephalad than the sensory block (pain, light touch), which, in turn, is usually several segments more cephalad than the motor blockade.
Differential blockade typically results in sympathetic blockade (judged by temperature sensitivity) that may be two segments or more cephalad than the sensory block (pain, light touch), which, in turn, is usually several segments more cephalad than the motor blockade.
 Interruption of efferent autonomic transmission at the spinal nerve roots during neuraxial blocks produces sympathetic blockade.
Interruption of efferent autonomic transmission at the spinal nerve roots during neuraxial blocks produces sympathetic blockade.
 Neuraxial blocks typically produce variable decreases in blood pressure that may be accompanied by a decrease in heart rate.
Neuraxial blocks typically produce variable decreases in blood pressure that may be accompanied by a decrease in heart rate.
 Deleterious cardiovascular effects should be anticipated and steps undertaken to minimize the degree of hypotension. However, volume loading with 10-20 mL/kg of intravenous fluid in a healthy patient before initiation of the block has been shown repeatedly to fail to prevent hypotension (in the absence of preexisting hypovolemia).
Deleterious cardiovascular effects should be anticipated and steps undertaken to minimize the degree of hypotension. However, volume loading with 10-20 mL/kg of intravenous fluid in a healthy patient before initiation of the block has been shown repeatedly to fail to prevent hypotension (in the absence of preexisting hypovolemia).
 Excessive or symptomatic bradycardia should be treated with atropine, and hypotension should be treated with vasopressors.
Excessive or symptomatic bradycardia should be treated with atropine, and hypotension should be treated with vasopressors.
 Major contraindications to neuraxial anesthesia include patient refusal, bleeding diathesis, severe hypovolemia, elevated intracranial pressure, and infection at the site of injection.
Major contraindications to neuraxial anesthesia include patient refusal, bleeding diathesis, severe hypovolemia, elevated intracranial pressure, and infection at the site of injection.
 For epidural anesthesia, a sudden loss of resistance (to injection of air or saline) is encountered as the needle passes through the ligamentum flavum and enters the epidural space. For spinal anesthesia, the needle is advanced through the epidural space and penetrates the dura-subarachnoid membranes, as signaled by freely flowing cerebrospinal fluid.
For epidural anesthesia, a sudden loss of resistance (to injection of air or saline) is encountered as the needle passes through the ligamentum flavum and enters the epidural space. For spinal anesthesia, the needle is advanced through the epidural space and penetrates the dura-subarachnoid membranes, as signaled by freely flowing cerebrospinal fluid.
 Continuous epidural anesthesia is a neuraxial technique offering a range of applications wider than the typical all-or-nothing, single dose spinal anesthetic. An epidural block can be performed at the lumbar, thoracic, or cervical level.
Continuous epidural anesthesia is a neuraxial technique offering a range of applications wider than the typical all-or-nothing, single dose spinal anesthetic. An epidural block can be performed at the lumbar, thoracic, or cervical level.
 Epidural techniques are widely used for surgical anesthesia, obstetric analgesia, postoperative pain control, and chronic pain management.
Epidural techniques are widely used for surgical anesthesia, obstetric analgesia, postoperative pain control, and chronic pain management.
 Epidural anesthesia is slower in onset (10-20 min) and may not be as dense as spinal anesthesia.
Epidural anesthesia is slower in onset (10-20 min) and may not be as dense as spinal anesthesia.
 The quantity (volume and concentration) of local anesthetic needed for epidural anesthesia is larger than that needed for spinal anesthesia. Toxic side effects are likely if a “full epidural dose” is injected intrathecally or intravascularly.
The quantity (volume and concentration) of local anesthetic needed for epidural anesthesia is larger than that needed for spinal anesthesia. Toxic side effects are likely if a “full epidural dose” is injected intrathecally or intravascularly.
 Caudal epidural anesthesia is a common regional technique in pediatric patients.
Caudal epidural anesthesia is a common regional technique in pediatric patients.
Spinal, Epidural, & Caudal Blocks: Introduction
Spinal, caudal, and epidural blocks were first used for surgical procedures at the turn of the twentieth century. These central blocks were widely used worldwide until reports of permanent neurological injury appeared, most prominently in the United Kingdom. However, a large-scale epidemiological study conducted in the 1950s indicated that complications were rare when these blocks were performed skillfully, with attention to asepsis, and when newer, safer local anesthetics were used. Today, neuraxial blocks are widely used for labor analgesia, caesarian section, orthopedic procedures, perioperative analgesia, and chronic pain management. However, they are still associated with various complications, and much literature has examined the incidence of complications following neuraxial blocks associated with different disease states. Additionally, various organizations continue to issue “guidelines” related to the management of regional anesthesia.
 Neuraxial anesthesia greatly expands the anesthesiologists’ armamentarium, providing alternatives to general anesthesia when appropriate. Neuraxial anesthesia may be used simultaneously with general anesthesia or afterward for postoperative analgesia. Neuraxial blocks can be performed as a single injection or with a catheter to allow intermittent boluses or continuous infusions.
Neuraxial anesthesia greatly expands the anesthesiologists’ armamentarium, providing alternatives to general anesthesia when appropriate. Neuraxial anesthesia may be used simultaneously with general anesthesia or afterward for postoperative analgesia. Neuraxial blocks can be performed as a single injection or with a catheter to allow intermittent boluses or continuous infusions.
Neuraxial techniques have proved to be safe when well managed; however, there is still a risk of complications. Adverse reactions and complications range from self-limited back soreness to debilitating permanent neurological deficits and even death. The practitioner must therefore have a good understanding of the anatomy involved, be thoroughly familiar with the pharmacology and toxic dosages of the agents employed, diligently employ sterile techniques, and anticipate and quickly treat physiological derangements.
Almost all operations at or below the neck have been performed under neuraxial anesthesia. Indeed, cardiac and thoracic surgeries have been performed in this manner. However, because intrathoracic, upper abdominal, and laparoscopic operations can significantly impair ventilation, general anesthesia with endotracheal intubation is usually necessary. So why perform a regional anesthetic for these cases, or for any other?
Some studies suggest that postoperative morbidity—and possibly mortality—may be reduced when neuraxial blockade is used either alone or in combination with general anesthesia. Neuraxial blocks may reduce the incidence of venous thrombosis and pulmonary embolism, cardiac complications in high-risk patients, bleeding and transfusion requirements, vascular graft occlusion, and pneumonia and respiratory depression following upper abdominal or thoracic surgery in patients with chronic lung disease. Neuraxial blocks may also allow earlier return of gastrointestinal function following surgery. Proposed mechanisms (in addition to avoidance of larger doses of anesthetics and opioids) include amelioration of the hypercoagulable state associated with surgery, sympathectomy-mediated increases in tissue blood flow, improved oxygenation from decreased splinting, enhanced peristalsis, and suppression of the neuroendocrine stress response to surgery. In patients with coronary artery disease, a decreased stress response may result in less perioperative ischemia and reduced morbidity and mortality. Reduction of parenteral opioid requirements may decrease the incidence of atelectasis, hypoventilation, and aspiration pneumonia and reduce the duration of ileus. Postoperative epidural analgesia may also significantly reduce both the time until extubation and the need for mechanical ventilation after major abdominal or thoracic surgery. Regional anesthesia may also preserve immunity perioperatively, reducing the risk of cancer spread according to some studies.
Anesthesiologists are all too familiar with situations in which a consultant “clears” a sick elderly patient with significant cardiac disease for surgery “under spinal anesthesia.” But, is a spinal anesthetic really safer than general anesthesia in such a patient? A spinal anesthetic with no intravenous sedation may reduce the likelihood of postoperative delirium or cognitive dysfunction, which is sometimes seen in the elderly. Unfortunately, some, if not most, patients require some sedation during the course of the procedure, either for comfort or to facilitate cooperation. Is spinal anesthesia always safer in a patient with severe coronary artery disease or a decreased ejection fraction? Ideally, an anesthetic technique in such a patient should not produce either hypotension (which decreases myocardial perfusion pressure) or hypertension and tachycardia (which increase myocardial oxygen consumption), and, also, should not require large fluid infusions (which can precipitate congestive heart failure). Spinal anesthesia can produce both hypotension and bradycardia, which may be rapid in onset and are sometimes profound. Moreover, treatment that includes rapid administration of intravenous fluid can cause fluid overload (when the vasodilatation wears off). The slower onset of hemodynamic responses to epidural anesthesia may give the anesthesiologist more time to correct these changes. General anesthesia, on the other hand, also poses potential problems for patients with cardiac compromise. Most general anesthetics are cardiac depressants, and many cause vasodilatation. Deep anesthesia can readily cause hypotension, whereas light anesthesia relative to the level of stimulation causes hypertension and tachycardia. Insertion of a laryngeal mask airway causes less of a stress response than does endotracheal intubation, but deeper levels of general anesthesia are still required to blunt the response to surgical stimulation.
Thus, arguments can be made for and against neuraxial and regional anesthesia in this setting. Perhaps then it is not the technique, per se, that is critical as much as the careful execution with appropriate monitoring and management of whatever anesthetic technique is planned.
Neuraxial anesthesia has had a great impact in obstetrics. Currently, epidural anesthesia is widely used for analgesia in women in labor and during vaginal delivery. Cesarean section is most commonly performed under epidural or spinal anesthesia. Both blocks allow a mother to remain awake and experience the birth of her child. Large population studies in Great Britain and the United States have shown that regional anesthesia for cesarean section is associated with less maternal morbidity and mortality than is general anesthesia. This may be largely due to a reduction in the incidence of pulmonary aspiration and failed intubation when neuraxial anesthesia is employed. Fortunately, the increased availability of video laryngoscopes may also reduce the incidence of adverse outcomes related to airway difficulties associated with general anesthesia for cesarean section.
Anatomy
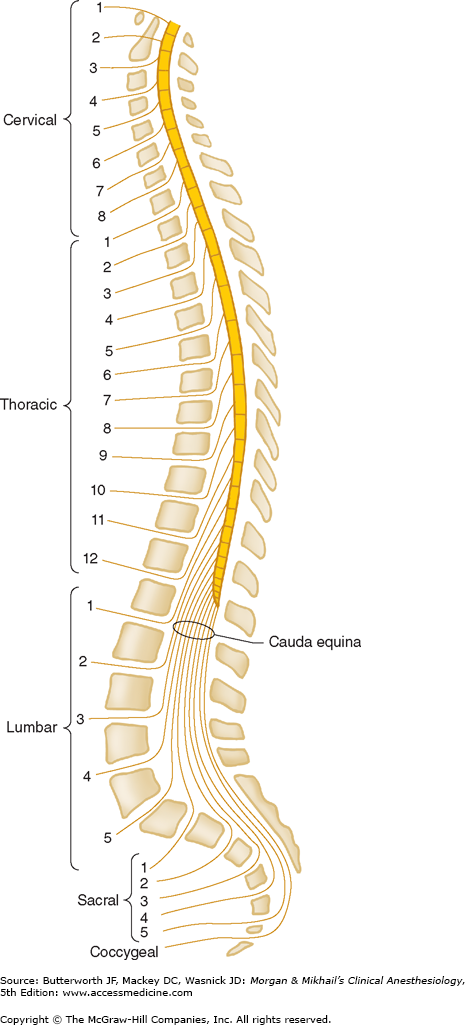
The spine is composed of the vertebral bones and intervertebral disks (Figure 45-1). There are 7 cervical (C), 12 thoracic (T), and 5 lumbar (L) vertebrae (Figure 45-2). The sacrum is a fusion of 5 sacral (S) vertebrae, and there are small rudimentary coccygeal vertebrae. The spine as a whole provides structural support for the body and protection for the spinal cord and nerves and allows a degree of mobility in several spatial planes. At each vertebral level, paired spinal nerves exit the central nervous system (Figure 45-2).
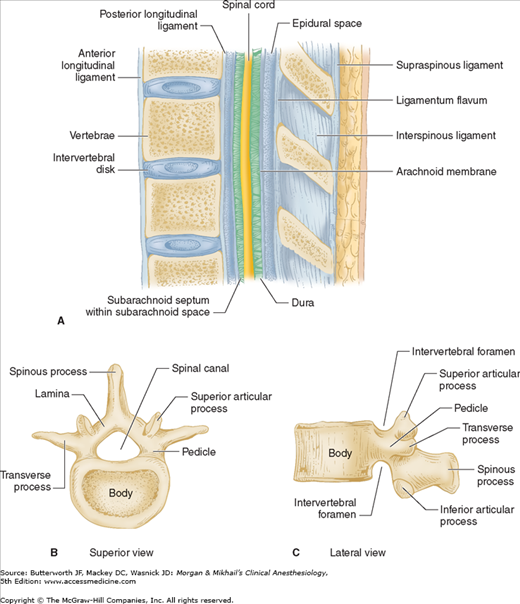
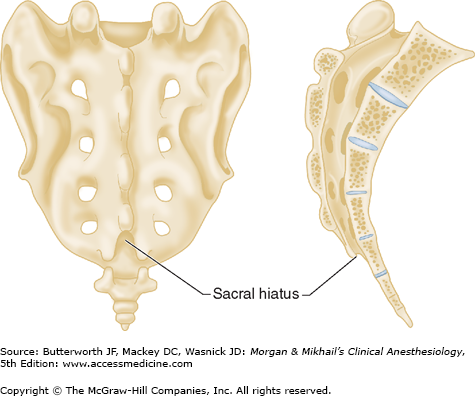
Vertebrae differ in shape and size at the various levels. The first cervical vertebra, the atlas, lacks a body and has unique articulations with the base of the skull and the second vertebra. The second vertebra, called the axis, consequently has atypical articulating surfaces. All 12 thoracic vertebrae articulate with their corresponding rib. Lumbar vertebrae have a large anterior cylindrical vertebral body. A hollow ring is defined anteriorly by the vertebral body, laterally by the pedicles and transverse processes, and posteriorly by the lamina and spinous processes (Figure 45-1B and C). The laminae extend between the transverse processes and the spinous processes, and the pedicle extends between the vertebral body and the transverse processes. When stacked vertically, the hollow rings become the spinal canal in which the spinal cord and its coverings sit. The individual vertebral bodies are connected by the intervertebral disks. There are four small synovial joints at each vertebra, two articulating with the vertebra above it and two with the vertebra below. These are the facet joints, which are adjacent to the transverse processes (Figure 45-1C). The pedicles are notched superiorly and inferiorly, these notches forming the intervertebral foramina from which the spinal nerves exit. Sacral vertebrae normally fuse into one large bone, the sacrum, but each one retains discrete anterior and posterior intervertebral foramina. The laminae of S5 and all or part of S4 normally do not fuse, leaving a caudal opening to the spinal canal, the sacral hiatus (Figure 45-3).
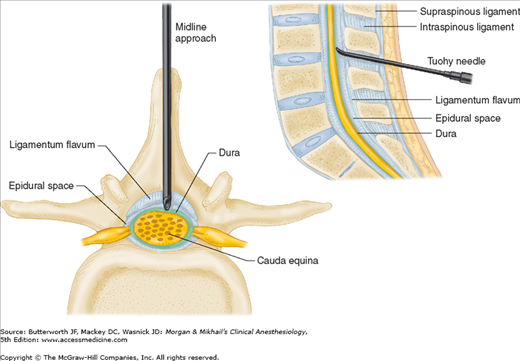
The spinal column normally forms a double C, being convex anteriorly in the cervical and lumbar regions (Figure 45-2). Ligamentous elements provide structural support, and, together with supporting muscles, help to maintain the unique shape. Ventrally, the vertebral bodies and intervertebral disks are connected and supported by the anterior and posterior longitudinal ligaments (Figure 45-1A). Dorsally, the ligamentum flavum, interspinous ligament, and supraspinous ligament provide additional stability. Using the midline approach, a needle passes through these three dorsal ligaments and through an oval space between the bony lamina and spinous processes of adjacent vertebra (Figure 45-4).
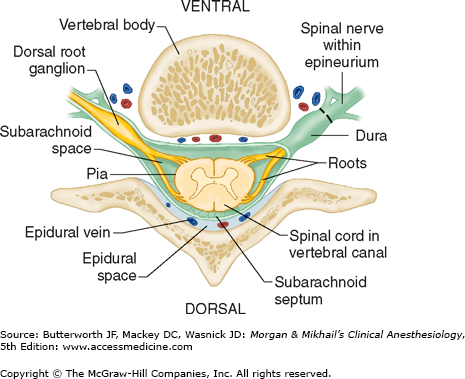
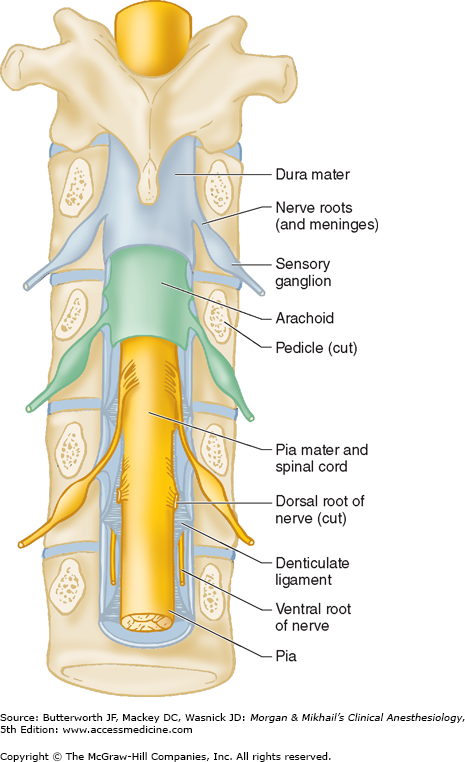
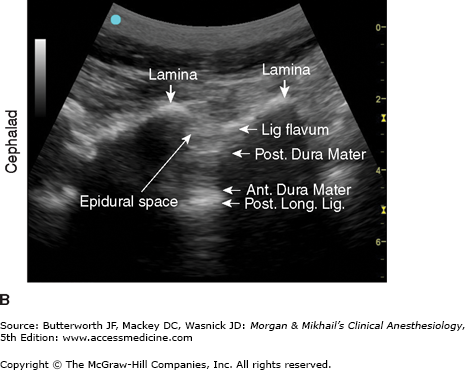
The spinal canal contains the spinal cord with its coverings (the meninges), fatty tissue, and a venous plexus (Figure 45-5). The meninges are composed of three layers: the pia mater, the arachnoid mater, and the dura mater; all are contiguous with their cranial counterparts (Figure 45-6). The pia mater is closely adherent to the spinal cord, whereas the arachnoid mater is usually closely adherent to the thicker and denser dura mater. Cerebrospinal fluid (CSF) is contained between the pia and arachnoid maters in the subarachnoid space. The spinal subdural space is generally a poorly demarcated, potential space that exists between the dura and arachnoid membranes. The epidural space is a better defined potential space within the spinal canal that is bounded by the dura and the ligamentum flavum (Figures 45-1 and 45-5).
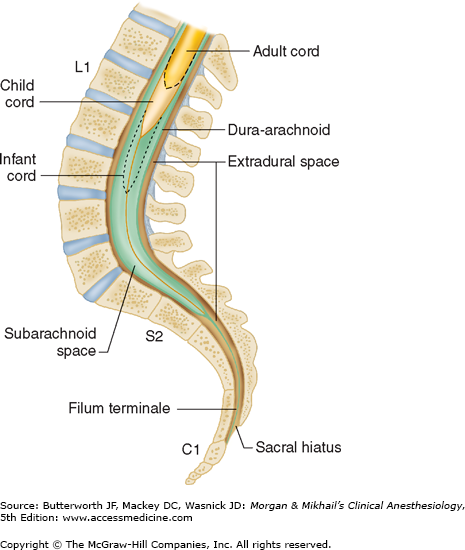
The spinal cord normally extends from the foramen magnum to the level of L1 in adults (Figure 45-7). In children, the spinal cord ends at L3 and moves up with age. The anterior and posterior nerve roots at each spinal level join one another and exit the intervertebral foramina, forming spinal nerves from C1 to S5 (Figure 45-2). At the cervical level, the nerves arise above their respective vertebrae, but starting at T1, exit below their vertebrae. As a result, there are eight cervical nerve roots, but only seven cervical vertebrae. The cervical and upper thoracic nerve roots emerge from the spinal cord and exit the vertebral foramina nearly at the same level (Figure 45-2). But, because the spinal cord normally ends at L1, lower nerve roots course some distance before exiting the intervertebral foramina. These lower spinal nerves form the cauda equina (“horse’s tail”; Figure 45-2).  Therefore, performing a lumbar (subarachnoid) puncture below L1 in an adult (L3 in a child) usually avoids potential needle trauma to the cord; damage to the cauda equina is unlikely, as these nerve roots float in the dural sac below L1 and tend to be pushed away (rather than pierced) by an advancing needle.
Therefore, performing a lumbar (subarachnoid) puncture below L1 in an adult (L3 in a child) usually avoids potential needle trauma to the cord; damage to the cauda equina is unlikely, as these nerve roots float in the dural sac below L1 and tend to be pushed away (rather than pierced) by an advancing needle.
A dural sheath invests most nerve roots for a small distance, even after they exit the spinal canal (Figure 45-5). Nerve blocks close to the intervertebral foramen therefore carry a risk of subdural or subarachnoid injection. The dural sac and the subarachnoid and subdural spaces usually extend to S2 in adults and often to S3 in children. Because of this fact and the smaller body size, caudal anesthesia carries a greater risk of subarachnoid injection in children than in adults. An extension of the pia mater, the filum terminale, penetrates the dura and attaches the terminal end of the spinal cord (conus medullaris) to the periosteum of the coccyx (Figure 45-7).
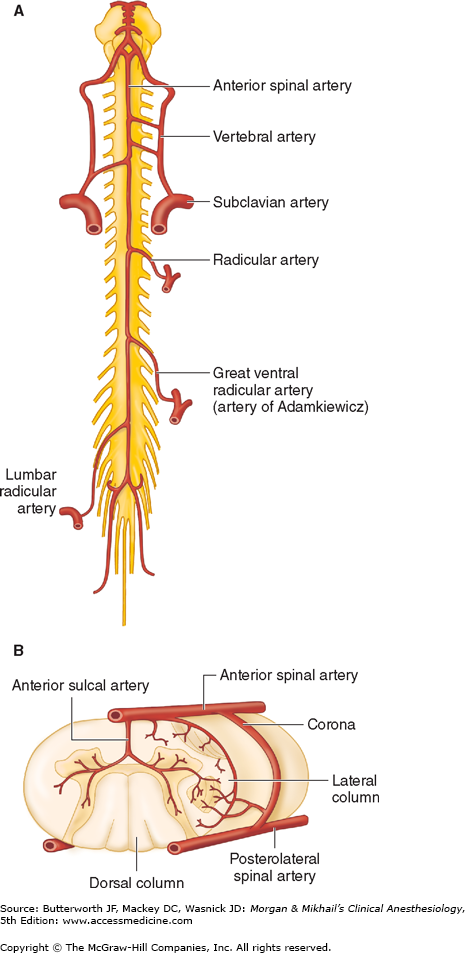
The blood supply to the spinal cord and nerve roots is derived from a single anterior spinal artery and paired posterior spinal arteries (Figure 45-8). The anterior spinal artery is formed from the vertebral artery at the base of the skull and courses down along the anterior surface of the cord. The anterior spinal artery supplies the anterior two-thirds of the cord, whereas the two posterior spinal arteries supply the posterior one-third. The posterior spinal arteries arise from the posterior inferior cerebellar arteries and course down along the dorsal surface of the cord medial to the dorsal nerve roots. The anterior and posterior spinal arteries receive additional blood flow from the intercostal arteries in the thorax and the lumbar arteries in the abdomen. One of these radicular arteries is typically large, the artery of Adamkiewicz, or arteria radicularis magna, arising from the aorta (Figures 45-8A). It is typically unilateral and nearly always arises on the left side, providing the major blood supply to the anterior, lower two-thirds of the spinal cord. Injury to this artery can result in the anterior spinal artery syndrome.
Figure 45-8
Arterial supply to the spinal cord. A: Anterior view showing principal sources of blood supply. B: Cross-sectional view through the spinal cord showing paired posterior spinal arteries and a single anterior spinal artery. (Adapted and reproduced, with permission, from Waxman SG: Correlative Neuroanatomy, 24th ed. McGraw-Hill, 2000.)
Mechanism of Action
 The mechanisms of spinal and epidural anesthesia remain speculative. The principal site of action for neuraxial blockade is believed to be the nerve root. Local anesthetic is injected into CSF (spinal anesthesia) or the epidural space (epidural and caudal anesthesia) and bathes the nerve root in the subarachnoid space or epidural space, respectively. Direct injection of local anesthetic into CSF for spinal anesthesia allows a relatively small dose and volume of local anesthetic to achieve dense sensory and motor blockade. In contrast, the same local anesthetic concentration is achieved within nerve roots only with much larger volumes and quantities of local anesthetic molecules during epidural and caudal anesthesia. Moreover, the injection site (level) for epidural anesthesia must generally be close to the nerve roots that must be anesthetized. Blockade of neural transmission (conduction) in the posterior nerve root fibers interrupts somatic and visceral sensation, whereas blockade of anterior nerve root fibers prevents efferent motor and autonomic outflow. Local anesthetics may also have actions on structures within the spinal cord during epidural and spinal anesthesia.
The mechanisms of spinal and epidural anesthesia remain speculative. The principal site of action for neuraxial blockade is believed to be the nerve root. Local anesthetic is injected into CSF (spinal anesthesia) or the epidural space (epidural and caudal anesthesia) and bathes the nerve root in the subarachnoid space or epidural space, respectively. Direct injection of local anesthetic into CSF for spinal anesthesia allows a relatively small dose and volume of local anesthetic to achieve dense sensory and motor blockade. In contrast, the same local anesthetic concentration is achieved within nerve roots only with much larger volumes and quantities of local anesthetic molecules during epidural and caudal anesthesia. Moreover, the injection site (level) for epidural anesthesia must generally be close to the nerve roots that must be anesthetized. Blockade of neural transmission (conduction) in the posterior nerve root fibers interrupts somatic and visceral sensation, whereas blockade of anterior nerve root fibers prevents efferent motor and autonomic outflow. Local anesthetics may also have actions on structures within the spinal cord during epidural and spinal anesthesia.
By interrupting the afferent transmission of painful stimuli and abolishing the efferent impulses responsible for skeletal muscle tone, neuraxial blocks can provide excellent operating conditions. Sensory blockade interrupts both somatic and visceral painful stimuli. The mechanism of action of local anesthetic agents is discussed in Chapter 16. The effect of local anesthetics on nerve fibers varies according to the size and characteristics of the nerve fiber, whether it is myelinated, the length of nerve that is bathed by the local anesthetic, and the concentration of the local anesthetic. Spinal nerve roots contain varying mixtures of these fiber types. Smaller and myelinated fibers are generally more easily blocked than larger and unmyelinated ones. The size and character of the fiber types, and the fact that the concentration of local anesthetic decreases with increasing distance from the level of injection, explains the phenomenon of differential blockade during neuraxial anesthesia.  Differential blockade typically results in sympathetic blockade (judged by temperature sensitivity) that may be two segments or more cephalad than the sensory block (pain, light touch), which, in turn, is usually several segments more cephalad than the motor blockade.
Differential blockade typically results in sympathetic blockade (judged by temperature sensitivity) that may be two segments or more cephalad than the sensory block (pain, light touch), which, in turn, is usually several segments more cephalad than the motor blockade.
 Interruption of efferent autonomic transmission at the spinal nerve roots during neuraxial blocks produces sympathetic blockade. Sympathetic outflow from the spinal cord may be described as thoracolumbar, whereas parasympathetic outflow is craniosacral. Sympathetic preganglionic nerve fibers (small, myelinated B fibers) exit the spinal cord with the spinal nerves from T1-L2 and may course many levels up or down the sympathetic chain before synapsing with a postganglionic cell in a sympathetic ganglion. In contrast, parasympathetic preganglionic fibers exit the spinal cord with the cranial and sacral nerves. Neuraxial anesthesia does not block the vagus nerve (tenth cranial nerve). The physiological responses of neuraxial blockade therefore result from decreased sympathetic tone and/or unopposed parasympathetic tone.
Interruption of efferent autonomic transmission at the spinal nerve roots during neuraxial blocks produces sympathetic blockade. Sympathetic outflow from the spinal cord may be described as thoracolumbar, whereas parasympathetic outflow is craniosacral. Sympathetic preganglionic nerve fibers (small, myelinated B fibers) exit the spinal cord with the spinal nerves from T1-L2 and may course many levels up or down the sympathetic chain before synapsing with a postganglionic cell in a sympathetic ganglion. In contrast, parasympathetic preganglionic fibers exit the spinal cord with the cranial and sacral nerves. Neuraxial anesthesia does not block the vagus nerve (tenth cranial nerve). The physiological responses of neuraxial blockade therefore result from decreased sympathetic tone and/or unopposed parasympathetic tone.
 Neuraxial blocks produce variable decreases in blood pressure that may be accompanied by a decrease in heart rate. These effects are generally proportional to the dermatomal level and extent of sympathectomy. Vasomotor tone is primarily determined by sympathetic fibers arising from T5-L1, innervating arterial and venous smooth muscle. Blocking these nerves causes vasodilation of the venous capacitance vessels and pooling of blood in the viscera and lower extremities, thereby decreasing the effective circulating blood volume and venous return to the heart. Arterial vasodilation may also decrease systemic vascular resistance. The effects of arterial vasodilation may be minimized by compensatory vasoconstriction above the level of the block, particularly when the extent of sensory anesthesia is limited to the lower thoracic dermatomes. A high sympathetic block not only prevents compensatory vasoconstriction, but may also block the sympathetic cardiac accelerator fibers that arise at T1-T4. Profound hypotension may result from arterial dilation and venous pooling combined with bradycardia (and possibly also milder degrees of decreased contractility). These effects are exaggerated if venous pooling is further augmented by a head-up position or the weight of a gravid uterus. Unopposed vagal tone may explain the sudden cardiac arrest sometimes seen with spinal anesthesia.
Neuraxial blocks produce variable decreases in blood pressure that may be accompanied by a decrease in heart rate. These effects are generally proportional to the dermatomal level and extent of sympathectomy. Vasomotor tone is primarily determined by sympathetic fibers arising from T5-L1, innervating arterial and venous smooth muscle. Blocking these nerves causes vasodilation of the venous capacitance vessels and pooling of blood in the viscera and lower extremities, thereby decreasing the effective circulating blood volume and venous return to the heart. Arterial vasodilation may also decrease systemic vascular resistance. The effects of arterial vasodilation may be minimized by compensatory vasoconstriction above the level of the block, particularly when the extent of sensory anesthesia is limited to the lower thoracic dermatomes. A high sympathetic block not only prevents compensatory vasoconstriction, but may also block the sympathetic cardiac accelerator fibers that arise at T1-T4. Profound hypotension may result from arterial dilation and venous pooling combined with bradycardia (and possibly also milder degrees of decreased contractility). These effects are exaggerated if venous pooling is further augmented by a head-up position or the weight of a gravid uterus. Unopposed vagal tone may explain the sudden cardiac arrest sometimes seen with spinal anesthesia.
 Deleterious cardiovascular effects should be anticipated and steps undertaken to minimize the degree of hypotension. However, volume loading with 10-20 mL/kg of intravenous fluid in a healthy patient before initiation of the block has been shown repeatedly to fail to prevent hypotension (in the absence of preexisting hypovolemia). Left uterine displacement in the third trimester of pregnancy helps to minimize physical obstruction to venous return. Despite these efforts, hypotension may still occur and should be treated promptly. Autotransfusion may be accomplished by placing the patient in a head-down position. A bolus of intravenous fluid (5-10 mL/kg) may be helpful in patients who have adequate cardiac and renal function to be able to “handle” the fluid load after the block wears off.
Deleterious cardiovascular effects should be anticipated and steps undertaken to minimize the degree of hypotension. However, volume loading with 10-20 mL/kg of intravenous fluid in a healthy patient before initiation of the block has been shown repeatedly to fail to prevent hypotension (in the absence of preexisting hypovolemia). Left uterine displacement in the third trimester of pregnancy helps to minimize physical obstruction to venous return. Despite these efforts, hypotension may still occur and should be treated promptly. Autotransfusion may be accomplished by placing the patient in a head-down position. A bolus of intravenous fluid (5-10 mL/kg) may be helpful in patients who have adequate cardiac and renal function to be able to “handle” the fluid load after the block wears off.  Excessive or symptomatic bradycardia should be treated with atropine, and hypotension should be treated with vasopressors. Direct α-adrenergic agonists (such as phenylephrine) primarily produce arteriolar constriction and may reflexively increase bradycardia, increasing systemic vascular resistance. The “mixed” agent ephedrine has direct and indirect β-adrenergic effects that increase heart rate and contractility and indirect effects that also produce vasoconstriction. Much like ephedrine, small doses of epinephrine (2-5 mcg boluses) are particularly useful in treating spinal anesthesia induced hypotension. If profound hypotension and/or bradycardia persist, vasopressor infusions may be required.
Excessive or symptomatic bradycardia should be treated with atropine, and hypotension should be treated with vasopressors. Direct α-adrenergic agonists (such as phenylephrine) primarily produce arteriolar constriction and may reflexively increase bradycardia, increasing systemic vascular resistance. The “mixed” agent ephedrine has direct and indirect β-adrenergic effects that increase heart rate and contractility and indirect effects that also produce vasoconstriction. Much like ephedrine, small doses of epinephrine (2-5 mcg boluses) are particularly useful in treating spinal anesthesia induced hypotension. If profound hypotension and/or bradycardia persist, vasopressor infusions may be required.
Alterations in pulmonary physiology are usually minimal with neuraxial blocks because the diaphragm is innervated by the phrenic nerve, with fibers originating from C3-C5. Even with high thoracic levels, tidal volume is unchanged; there is only a small decrease in vital capacity, which results from a loss of the abdominal muscles’ contribution to forced expiration.
Patients with severe chronic lung disease may rely upon accessory muscles of respiration (intercostal and abdominal muscles) to actively inspire or exhale. High levels of neural blockade will impair these muscles. Similarly, effective coughing and clearing of secretions require these muscles for expiration. For these reasons, neuraxial blocks should be used with caution in patients with limited respiratory reserve. These deleterious effects need to be weighed against the advantages of avoiding airway instrumentation and positive-pressure ventilation. For surgical procedures above the umbilicus, a pure regional technique may not be the best choice in patients with severe lung disease. On the other hand, these patients may benefit from the effects of thoracic epidural analgesia (with dilute local anesthetics and opioids) in the postoperative period, particularly following upper abdominal or thoracic surgery. Some evidence suggests that postoperative thoracic epidural analgesia in high-risk patients can improve pulmonary outcome by decreasing the incidence of pneumonia and respiratory failure, improving oxygenation, and decreasing the duration of mechanical ventilatory support.
Sympathetic outflow originates at the T5-L1 level. Neuraxial block-induced sympathectomy allows vagal tone dominance and results in a small, contracted gut with active peristalsis. This can improve operative conditions during laparoscopy when used as an adjunct to general anesthesia. Postoperative epidural analgesia with local anesthetics and minimal systemic opioids hastens the return of gastrointestinal function after open abdominal procedures.
Hepatic blood flow will decrease with reductions in mean arterial pressure from any anesthetic technique, including neuraxial anesthesia.
Renal blood flow is maintained through autoregulation, and there is little effect of neuraxial anesthesia on renal function. Neuraxial anesthesia at the lumbar and sacral levels blocks both sympathetic and parasympathetic control of bladder function. Loss of autonomic bladder control results in urinary retention until the block wears off. If no urinary catheter is placed perioperatively, it is prudent to use the regional anesthetic of shortest duration sufficient for the surgical procedure and to administer the minimal safe volume of intravenous fluid. Patients with urinary retention should be checked for bladder distention after neuraxial anesthesia.
Surgical trauma produces a systemic neuroendocrine response via activation of somatic and visceral afferent nerve fibers, in addition to a localized inflammatory response. This systemic response includes increased concentrations of adrenocorticotropic hormone, cortisol, epinephrine, norepinephrine, and vasopressin levels, as well as activation of the renin-angiotensin-aldosterone system. Clinical manifestations include intraoperative and postoperative hypertension, tachycardia, hyperglycemia, protein catabolism, suppressed immune responses, and altered renal function. Neuraxial blockade can partially suppress (during major invasive surgery) or totally block (during lower extremity surgery) the neuroendocrine stress response. To maximize this blunting of the neuroendocrine stress response, neuraxial block should precede incision and continue into the postoperative period.
Clinical Considerations Common to Spinal & Epidural Blocks
Neuraxial blocks may be used alone or in conjunction with general anesthesia for most procedures below the neck. Indeed, in some centers outside of North America, minimally invasive coronary artery surgery has been performed with thoracic epidural anesthesia alone. As a primary anesthetic, neuraxial blocks have proved most useful in lower abdominal, inguinal, urogenital, rectal, and lower extremity surgery. Lumbar spinal surgery may also be performed under spinal anesthesia. Upper abdominal procedures (eg, gastrectomy) have been performed with spinal or epidural anesthesia, but because it can be difficult to safely achieve a sensory level adequate for patient comfort, these techniques are not commonly used.
If a neuraxial anesthetic is being considered, the risks and benefits must be discussed with the patient, and informed consent should be obtained. The patient must be mentally prepared for neuraxial anesthesia, and neuraxial anesthesia must be appropriate for the type of surgery. Patients should understand that they will have little or no lower extremity motor function until the block resolves. Procedures that require maneuvers that might compromise respiratory function (eg, pneumoperitoneum or pneumothorax) or are unusually prolonged are typically performed with general anesthesia, with or without neuraxial blockade.
 Major contraindications to neuraxial anesthesia include patient refusal, bleeding diathesis, severe hypovolemia, elevated intracranial pressure (particularly with an intracranial mass), and infection at the site of injection. Other relative contraindications include severe aortic or mitral stenosis and severe left ventricular outflow obstruction (hypertrophic obstructive cardiomyopathy); however, with close monitoring and control of the anesthetic level, neuraxial anesthesia can be performed safely in patients with valvular heart disease, particularly if extensive dermatomal spread of anesthesia is not required (eg, “saddle” block spinal anesthetics).
Major contraindications to neuraxial anesthesia include patient refusal, bleeding diathesis, severe hypovolemia, elevated intracranial pressure (particularly with an intracranial mass), and infection at the site of injection. Other relative contraindications include severe aortic or mitral stenosis and severe left ventricular outflow obstruction (hypertrophic obstructive cardiomyopathy); however, with close monitoring and control of the anesthetic level, neuraxial anesthesia can be performed safely in patients with valvular heart disease, particularly if extensive dermatomal spread of anesthesia is not required (eg, “saddle” block spinal anesthetics).
Relative and controversial contraindications are also shown in Table 45-1. Inspection and palpation of the back can reveal surgical scars, scoliosis, skin lesions, and whether the spinous processes can be identified. Although preoperative screening tests are not required in healthy patients undergoing neuraxial blockade, appropriate testing should be performed if the clinical history suggests a bleeding diathesis. Neuraxial anesthesia in the presence of sepsis or bacteremia could theoretically predispose patients to hematogenous spread of the infectious agents into the epidural or subarachnoid space, as has been shown for lumbar puncture in the presence of septicemia.
Absolute
|
Relative
|
Controversial
|
Patients with preexisting neurological deficits or demyelinating diseases may report worsening symptoms following a block. It may be impossible to discern effects or complications of the block from preexisting deficits or unrelated exacerbation of preexisting disease. For these reasons, some risk-averse practitioners argue against neuraxial anesthesia in such patients. A preoperative neurological examination should thoroughly document any deficits. In a retrospective study examining the records of 567 patients with preexisting neuropathies, 2 of the patients developed new or worsening neuropathy following neuraxial anesthesia. Although this finding indicates a relatively low risk of further injury, study investigators suggest that an injured nerve is vulnerable to additional injury, increasing the likelihood of poor neurological outcomes.
Regional anesthesia requires at least some degree of patient cooperation. This may be difficult or impossible for patients with dementia, psychosis, or emotional instability. The decision must be individualized. Unsedated young children may not be suitable for pure regional techniques; however, regional anesthesia is frequently used with general anesthesia in children.
Whether a block should be performed in the setting of anticoagulants and antiplatelet agents can be problematic. The American Society of Regional Anesthesia and Pain Medicine (ASRA) has issued several guidelines on this subject. Because guidelines are frequently revised and updated, practitioners are advised to seek the most recent edition. Although the incidence of epidural hematoma is reported to be quite low (1 in 150,000 epidurals), ASRA is concerned that the actual incidence may be somewhat higher. Moreover, the use of anticoagulant and antiplatelet medications continues to increase, placing an ever larger number of patients at potential risk of epidural hematomas. Because of the rarity of epidural hematomas, most guidelines are based on expert opinion and case series reviews, as clinical trials are not feasible.
If neuraxial anesthesia is to be used in patients receiving warfarin therapy, a normal prothrombin time and international normalized ratio should be documented prior to the block. Anesthesia staff should always consult with the patient’s primary physicians whenever considering the discontinuation of antiplatelet or antithrombotic therapy.
By themselves, aspirin and other nonsteroidal antiinflammatory drugs (NSAIDs) drugs do not increase the risk of spinal hematoma from neuraxial anesthesia procedures or epidural catheter removal. This assumes a normal patient with a normal coagulation profile who is not receiving other medications that might affect clotting mechanisms. In contrast, more potent agents should be stopped, and neuraxial blockade should generally be administered only after their effects have worn off. The waiting period depends on the specific agent: for ticlopidine (Ticlid), it is 14 days; clopidogrel (Plavix), 7 days; abciximab (Rheopro), 48 hr; and eptifibatide (Integrilin), 8 hr. In patients with a recently placed cardiac stent, discontinuation of antiplatelet therapy can result in stent thrombosis and acute ST-segment elevation myocardial infarction. Risks versus benefits of a neuraxial technique should be discussed with the patient and the patient’s primary doctors.
“Minidose” subcutaneous heparin prophylaxis is not a contraindication to neuraxial anesthesia or epidural catheter removal. In patients who are to receive systemic heparin intraoperatively, blocks may be performed 1 hr or more before heparin administration. A bloody epidural or spinal does not necessarily require cancellation of surgery, but discussion of the risks with the surgeon and careful postoperative monitoring is needed. Removal of an epidural catheter should occur 1 hr prior to, or 4 hr following, subsequent heparin dosing.
Neuraxial anesthesia should be avoided in patients on therapeutic doses of heparin and with increased partial thromboplastin time. If the patient is started on heparin after the placement of an epidural catheter, the catheter should be removed only after discontinuation or interruption of heparin infusion and evaluation of the coagulation status. The risk of spinal hematoma (with or without neuraxial puncture) is unclear in the setting of full anticoagulation for cardiac surgery. Prompt diagnosis and evacuation of symptomatic epidural hematomas increase the likelihood that neuronal function will be preserved.
Many cases of spinal hematoma associated with neuraxial anesthesia followed the introduction of the “low-molecular weight heparin” (LMWH) enoxaparin (Lovenox) in the United States in 1993. Many of these cases involved intraoperative or early postoperative LMWH use, and several patients were receiving concomitant antiplatelet medication. If an unusually bloody needle or catheter placement occurs, LMWH should be delayed until 24 hr postoperatively, because this trauma may increase the risk of spinal hematoma. If postoperative LMWH thromboprophylaxis will be utilized, epidural catheters should be removed 2 hr prior to the first LMWH dose. If already present, the catheter should be removed at least 10 hr after a dose of LMWH, and subsequent dosing should not occur for another 2 hr.
Neuraxial anesthesia should not be performed if a patient has received fibrinolytic or thrombolytic therapy.
Should lumbar neuraxial anesthesia, when used in conjunction with general anesthesia, be performed before or after induction of general anesthesia? This is controversial. The major arguments for having the patient asleep are that (1) most patients, if given a choice, would prefer to be asleep, and (2) the possibility of sudden patient movement causing injury is markedly diminished. The major argument for neuraxial blockade while the patient is still awake is that the patient can alert the clinician to paresthesias and pain on injection, both of which have been associated with postoperative neurological deficits. Although many clinicians are comfortable performing lumbar epidural or spinal puncture in anesthetized or deeply sedated adults, there is greater consensus that thoracic and cervical punctures should, except under unusual circumstances, only be performed in awake patients. Pediatric neuraxial blocks, particularly caudal and epidural blocks, are usually performed under general anesthesia.
Neuraxial blocks should be performed only in a facility in which all the equipment and drugs needed for intubation, resuscitation, and general anesthesia are immediately available. Regional anesthesia is greatly facilitated by adequate patient premedication. Nonpharmacologic patient preparation is also very helpful. The patient should be told what to expect so as to minimize anxiety. This is particularly important in situations in which premedication is not used, as is typically the case in obstetric anesthesia. Supplemental oxygen via a face mask or nasal cannula may be required to avoid hypoxemia when sedation is used. Minimum monitoring requirements include blood pressure and pulse oximetry for labor analgesia. Monitoring for blocks rendered in surgical anesthesia is the same as that in general anesthesia. Epidural steroid injections for management of pain (when little or no local anesthetic is injected) do not require continuous monitoring.
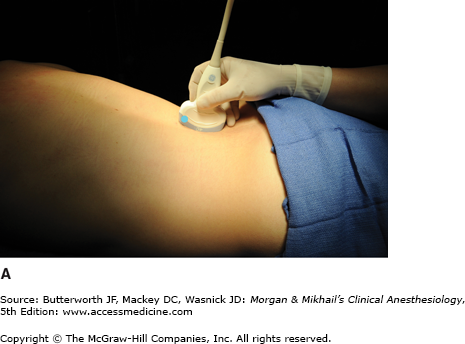
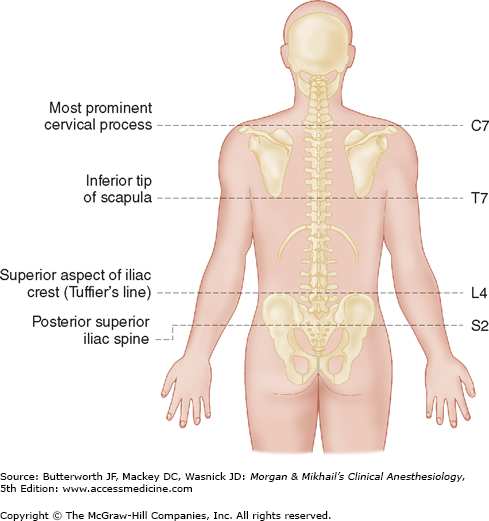
Spinous processes are generally palpable and help to define the midline. Ultrasound can be used when landmarks are not palpable (Figure 45-9). The spinous processes of the cervical and lumbar spine are nearly horizontal, whereas those in the thoracic spine slant in a caudal direction and can overlap significantly (Figure 45-2). Therefore, when performing a lumbar or cervical epidural block (with maximum spinal flexion), the needle is directed with only a slight cephalad angle, whereas for a thoracic block, the needle must be angled significantly more cephalad to enter the thoracic epidural space. In the cervical area, the first palpable spinous process is that of C2, but the most prominent one is that of C7 (vertebra prominens). With the arms at the side, the spinous process of T7 is usually at the same level as the inferior angle of the scapulae (Figure 45-10). A line drawn between the highest points of both iliac crests (Tuffier’s line) usually crosses either the body of L4 or the L4-L5 interspace. Counting spinous processes up or down from these reference points identifies other spinal levels. A line connecting the posterior superior iliac spine crosses the S2 posterior foramina. In slender persons, the sacrum is easily palpable, and the sacral hiatus is felt as a depression just above or between the gluteal clefts and above the coccyx, defining the point of entry for caudal blocks.
Figure 45-9
A: Transducer position to image paramedian epidural space at the lumbar spine, longitudinal view. B: Corresponding ultrasound image. Post. Long. Lig., Posterior Longitudinal Ligament; Lig. Flavum, Ligamentum Flavum; Ant. Dura Mater, Anterior Dura Mater; Post. Dura Mater, Posterior Dura Mater. (Reproduced, with permission, from Hadzic, A: Peripheral Nerve Blocks and Anatomy for Ultrasound-Guided Regional Anesthesia, 2nd edition. McGraw-Hill, 2012.)
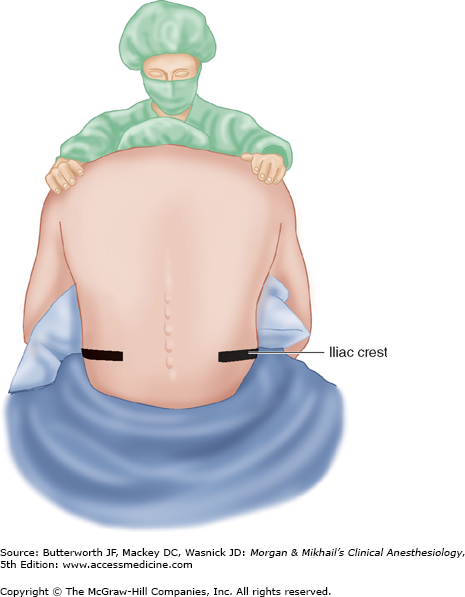
The anatomic midline is often easier to appreciate when the patient is sitting than when the patient is in the lateral decubitus position (Figure 45-11). This is particularly true with very obese patients. Patients sit with their elbows resting on their thighs or a bedside table, or they can hug a pillow. Flexion of the spine (arching the back “like a mad cat” maximizes the “target” area between adjacent spinous processes and brings the spine closer to the skin surface (Figure 45-12).