Complication
Frequency (%)
Electrode migration
1.5–13.2
Hardware malfunction
• Electrode
• IPG
3–9
3–25
Infection
2.5–14
Cerebrospinal fluid leakage
0.3–8
Pain at the incisions, IPG site
0.9–12
Subcutaneous hematoma or seroma
9
Epidural hematoma
4
Electrode fracture
Intraoperative neurologic injury
Case reports
Unexplained temporary paralysis
1.8
Others (e.g., skin erosion, aseptic meningitis, allergic dermatitis, activation of pyramidal tract)
Case reports
38.2.4 Surgical Complications
Complications may be avoided or at least diminished by good surgical technique and strict sterile precautions, as well as optimizing patient selection before the implantation according to published recommendations [20].
38.2.4.1 Seroma and CSF Hygromas
Seromas and hematomas can occur in up to 9% of patients with SCS implants [20]. Seromas, a collection of serous fluid beneath the wound, are one of the most benign surgical complications. Early detection is key in order to prevent infection complicating a seroma. Seromas present early after surgery similarly to hematomas with acute, afebrile, swelling and pain at the surgical site. If the seroma is very tender or large, it can be aspirated under sterile conditions. Empiric antibiotics are not recommended for seromas to avoid complicating the diagnosis: seromas can be managed conservatively with abdominal binder and serial aspirations if necessary, whereas infected hardware warrants explantation [17].
CSF leakage can occur in 0.3–7% of patients [20]. If the fluid collection is due to CSF accumulation (hygroma), the initial care is to observe and treat similarly as a seroma, but if the wound is tense and painful, it should be aspirated under sterile conditions. If laboratory analysis of the aspirate is consistent with CSF and the hygroma does not respond to conservative management, an epidural blood patch can be performed near the site of catheter entry into the intrathecal space to theoretically seal the leak. Rarely, referral for surgical exploration is necessary for large or persistent hygromas. If the patient has systemic symptoms and signs of infection and hygroma is present, urgent evaluation and treatment for meningitis should be initiated [17].
38.2.4.2 Hematoma
A hematoma is a blood collection in the subcutaneous tissues. They are associated with an increased risk of infection compared to seromas [17], which can best be mitigated by careful surgical technique and meticulous attention to hemostasis, as well as appropriate perioperative management of coagulation issues; any anticoagulant should be held an appropriate amount of time prior to surgery to aid in their prevention. There is a greater risk in exploring a small, stable hematoma compared to watchful waiting, as usually a hematoma will usually resolve on its own. Larger volume or expanding hematomas should be evacuated under sterile conditions to prevent wound dehiscence [17]. Basic laboratory studies of aspirated fluid and ultrasound imaging can be helpful to differentiate between seroma, hematoma, and infection.
38.2.4.3 Wound Dehiscence
Wound dehiscence (Fig. 38.1) occurs when one or more layers of the surgical wound separate. This most often occurs between 5 and 8 days after surgery [17]. It is more common in patients prone to poor wound healing, such as patients with diabetes, immunosuppression, and cancer. Wound closure with excessive tension on the wound itself can lead to ischemia and subsequent separation of tissue layers due to necrosis. Failure to sufficiently close tissue layers will also lead to dehiscence. In the absence of infection, the patient with a partially dehisced wound can be managed conservatively with regular decontamination of the wound and dressing changes to allow the wound to heal by secondary intention.
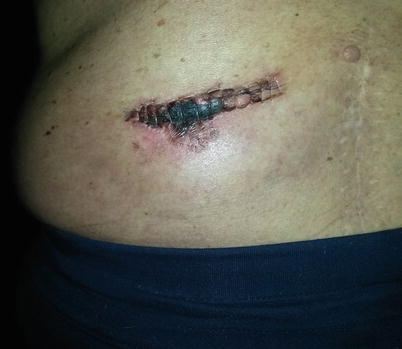

Fig. 38.1
Postoperative wound dehiscence. Photo courtesy of the University of California San Diego Anesthesia Department
38.2.4.4 Infection or Epidural Abscess
Infection or epidural abscess after an SCS trial or implantation calls for explantation of the device as well as decompression and drainage of the abscess. Infection rates after SCS implantation range from 2.5 to 14% [21]. The symptoms of an epidural abscess can be similar to an epidural hematoma, including fever, new neurologic deficits, leukocytosis, and severe pain and are all strong indications for emergent surgical decompression. Suspicion of an epidural abscess warrants an emergent CT scan and an infectious laboratory workup, including ESR, CRP, CBC, and blood cultures.
A recent international survey examined current reported infection control practices for SCS trials and implants and compared them to evidence-based recommendations obtained from standard surgical guidelines and recommendations of the Centers of Disease Control and Prevention (CDC), the National Institute for Health and Care Excellence (NICE), and the Surgical Care Improvement Project (SCIP) [24]. The authors identified multiple areas with high levels of noncompliance, including weight-based antibiotic dosing, hair removal strategies, double gloving, surgical dressing, skin antiseptic agent selection, and postoperative continuation of antibiotics [24]. Improved compliance with established infection control practices can significantly reduce reported rates of infection after SCS procedures.
38.2.4.5 Dural Puncture and Spinal Cord Injury
Although dural puncture is a very rare complication of SCS, it can occur, as noted in a case study of electrode placement into the spinal cord itself causing tetraparesis in a patient [25]. One of the most common causes of a “wet tap” is an unrecognized blood clot obstructing the lumen of the Tuohy needle during lead placement. With a clot in the needle, it is difficult to appreciate the loss of resistance when entering the epidural space. If blood drips back out of the hub, the physician should stop, flush the needle with saline, and withdraw the needle to make sure it is patent prior to proceeding [17]. Judicious use of multiple fluoroscopic angles (AP, lateral, and oblique), cautious needle advancement, and entering the epidural space below the termination of the spinal cord can help to prevent inadvertent dural puncture and potential spinal cord injury.
38.2.5 Hardware Complications
Although the available SCS systems are very reliable and most hardware malfunctions can be readily corrected, device malfunctions and complications should not be trivialized, as surgery is still required to repair them. Complications also interfere with the patient’s pain therapy, are costly, and are associated with all the attendant risks of surgery and anesthesia.
38.2.5.1 Lead Migration
After epidural placement, percutaneous leads are typically secured into the prevertebral fascia. Nonetheless, lead migration commonly occurs after implant, resulting not just in loss of analgesia but also potential for onset of new pain due to electrical stimulation of other structures, including the ligamentum flavum and/or the dorsal root entry zone (DREZ) [26]. Percutaneous spinal cord stimulation (SCS) electrodes are prone to migration even after scar tissue encapsulation [27] and are the most common mechanical reason for SCS failure [28]. In a retrospective review that examined records of SCS implantation between 2008 and 2011, 2.1% of the patients required a surgical revision due to clinically significant lead migration. Some investigators have estimated a 13–22% revision rate due to lead migration [29], with others reporting up to 30–40% [30]. Proper anchoring of the lead will lessen the chance of lead migration. Different manufacturers provide several different types of lead anchors; a simple “figure-of-eight” anchoring suture tie is also effective for securing stimulation leads [17]. A recent retrospective review of a novel fixation device demonstrated no lead migration at extended follow-up (10–68 weeks), suggesting that these types of devices may reduce the incidence of lead migration [30]. Another group showed the utility of bone cement to prevent lead migration with minimally invasive placement of spinal cord stimulator leads via laminectomy [31] (Fig. 38.2).
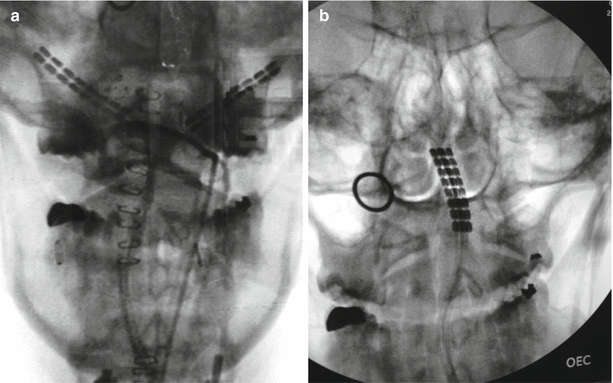

Fig. 38.2




Migration of occipital nerve stimulator leads. Panel a: proper intraoperative positioning of leads. Panel b: postoperative lead migration. Photo courtesy of the University of California San Diego Anesthesia Department
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






