Name of anti-TNF drug
Structure/mechanism of action
Infliximab
Chimeric (mouse/human) anti-TNF-alpha antibody
Etanercept
TNF-alpha receptor fusion protein
Adalimumab
Human TNF-alpha monoclonal anti-TNF-alpha antibody
Certolizumab pegol
Antigen-binding fragment (Fab) of human monoclonal antibody bound to polyethylene glycol
Golimumab
Human TNF-alpha monoclonal anti-TNF-alpha antibody
Perhaps the most serious acute complication of treating patients with TNF-alpha inhibitors is the development of life-threatening infection. The risk of tuberculosis (TB) (primary infection or reactivation) is the most notorious infectious risk for TNF-alpha inhibitors. The association was noted a few years after the initial approval of infliximab in 1998 [5]. These biologics carry an FDA black box warning for physicians and patients, and current recommendations are to perform a chest X-ray and tuberculin skin test prior to and during the administration of these medications [6].
In a meta-analysis of nine randomized clinical trials of anti-TNF-alpha therapy (infliximab or adalimumab) in rheumatoid arthritis (RA), there was a reported 2.0 odds ratio for serious infection as compared to placebo [7]. This is similar to what was found with the German biologics register where the relative risk of serious infection was 2.2 for etanercept and 2.1 for infliximab compared to disease-modifying antirheumatic drugs (DMARDs) [8]. In an earlier study, a total of 60 charts were reviewed of patients with RA for 2 years prior to the start of anti-TNF-alpha therapy and for about 1 year during treatment. The incidence of serious infection preceding therapy was 0.008 versus 0.181 per anti-TNF-alpha treatment year [9].
Analysis of the comprehensive national registry data of RA patients in the United Kingdom (UK) had differing results [10]. They compared the risk of serious infection in 8659 patients treated with anti-TNF-alpha agents with that of 2170 patients treated with traditional DMARDs. The rate of serious infection was 3.9 per 100 person-years in the DMARD cohort and 5.5 per 100 person-years in the anti-TNF-alpha group. However, after the adjustment for multiple comorbidities, sex and age, there was no significant difference in risk of infection between any of the anti-TNF-alpha cohorts and the comparison cohort.
This analysis compared the risk between the cohorts based upon an assumption of constant risk over time. This assumption has been shown to be incorrect [11]. Fu et al. analyzed the same database from the UK and found that the infection risk reaches a peak within the first month of use and then declines over the next 2 years until “stabilizing” [11]. Their analysis also suggests that patients are still at risk of developing serious infections outside of the “lag window” (the time after a patient is taken off of a drug but is still exposed to the effects of its therapy). In the case of most TNF-alpha inhibitors, this lag window is five half-lives, or 90 days.
Epidural abscess in patients on TNF-alpha inhibitors has been reported with patients on etanercept and infliximab; however, none of the reported cases were associated with epidural steroid injection [6, 12–15]. Three of the cases had no primary infection site where as one was thought secondary to a dental cleaning [12] and another from hematogenous seeding from a septic joint and cellulitis [13]. Epidural abscess is a rarely reported complication of epidural steroid injections, and the incidence remains undetermined [16]. With the paucity of reports of epidural abscess in patients on TNF-alpha inhibitors, we cannot recommend to abstain from injection therapy. However, one may consider postponing injection therapy in the setting of a patient recently starting an anti-TNF drug, as in the case of our patient.
18.2.2 Spinal Epidural Abscess
18.2.2.1 Epidemiology and Risk Factors
Spinal epidural abscess (SEA) is a surgical emergency that requires prompt diagnosis and treatment. It is generally a pyogenic infection and space-occupying lesion in the epidural space that causes neurologic sequel, including pain, paresthesias, paralysis, and even death. Bacteria can access the epidural space by direct extension from infected surroundings or, more commonly, from hematogenous seeding [17]. Abscesses can be located in the anterior or posterior space causing neurologic compromise from either direct compression or thrombosis of the vasculature. Spontaneous epidural abscess is rare, accounting for 0.2–2 cases per 10,000 hospital admissions per year [18, 19]. The incidence after central nerve block is quite varied and reported as 1:1000–1:100,000 [19]. In the literature, there is a definite prevalence for males with a male/female ratio of 1:0.56 [18].
In a meta-analysis of 915 patients with SEA, only 6% were attributed to either epidural anesthesia, injections in the epidural space, or spinal anesthesia [18]. The majority of patients will present with an identifiable source such as skin and soft tissue infections, indwelling catheters, frequent venous puncture, or spinal trauma/procedure.
Patients at increased risk of infection are more likely to develop an epidural abscess. Known risk factors for spinal epidural abscess are [18]:
- 1.
Compromised immunity: diabetes mellitus, immunosuppressive therapy, malignancy, pregnancy, HIV infection, cirrhosis, and alcohol abuse
- 2.
Disruption of the spinal column: degenerative disk disease, surgery, neuraxial blocks, and blunt trauma
- 3.
Source of infection: respiratory, urinary tract, soft tissue, IV drug users, and patients with indwelling catheters
18.2.2.2 Clinical Manifestations
As with many infectious processes, the initial manifestations of a spinal epidural abscess can be vague and nonspecific, presenting as malaise and fever. The diagnosis can be a challenge as it is quite rare among patients presenting with back pain. In a retrospective review of 46 patients who were diagnosed with SEA over a 10-year period, 89% had spinal pain, 67% had fever and chills, 57% had radicular pain, 37% had bowel or bladder dysfunctions, and 80% had paralysis (paraparesis, paraplegia) [20]. In an analysis of 871 patients, 71% had back pain, 66% had fever, 24% had incontinence, and 31% had paraplegia/paraparesis [18]. The combination of severe back pain as well as fever should be regarded as an early warning of SEA.
18.2.2.3 Clinical Examination
Fever and pain on palpation or) percussion may be noted. The neurological exam can be variable as the epidural abscess progresses. The clinical exam should include muscle strength testing, sensory testing, reflexes, and rectal exam.
18.2.2.4 Laboratory Studies
The laboratory findings that are markers for severe inflammation or infection may accompany SEA; however, they are not all specific: leukocytosis, erythrocyte sedimentation rate (ESR), C-reactive protein (CPR), and thrombocytopenia . Patients presenting with an abscess will frequently have a leukocytosis [18, 20]. In a prospective study in Emergency Department patients, Davis et al. found that a treatment algorithm that incorporated risk factor assessment followed by erythrocyte sedimentation rate (ESR) and c-reactive protein (CRP) testing was highly sensitive and moderately specific for identifying patients with SEA [21]. They found the sensitivity and specificity for those with risk factors pointing toward SEA to be 100% and 67%, respectively. The mean CRP level was significantly higher in SEA patients than those with risk factor who were non-SEA. They found the rate of delayed diagnosis drops 80% after the implementation of this protocol.
While thrombocytopenia is not sensitive or specific for the diagnosis, it may be a risk factor for poor outcome [20]. The low platelet count, as seen in our patient, implies sepsis and the commencement of disseminated intravascular coagulation, thus more likely resulting in greater morbidity.
Lumbar puncture comes with significant risk, such as spread of the infection to the CSF and meninges. While it could yield information from the analysis of the CSF or even pus, radiologic imaging is preferred [19].
18.2.2.5 Radiologic Studies
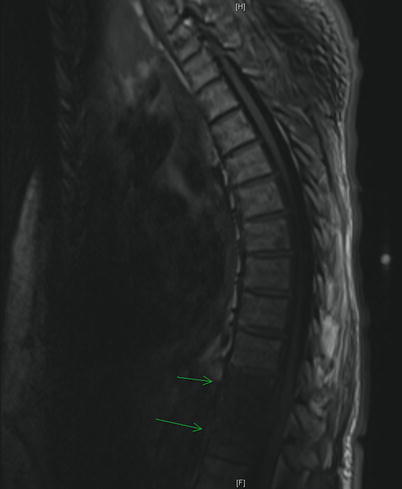
The plain film yields little utility in the initial evaluation of SEA, providing useful information in about 20% of cases [19]. However, abnormalities of the end plate should not be ignored as they can point toward an associated osteomyelitis [22]. A myelogram will reliably demonstrate a space-occupying lesion, but the same risks of lumbar puncture apply. While the computed tomography (CT) scan used to be the imaging modality of choice, the MRI is now the gold standard. Some recommend a full spinal MRI in the evaluation of suspected SEA [23]. The sensitivity of MRI is 91% compared to 92% with CT myelography [18]. However, MRI can be done without intervention and in all planes without moving a patient who may have neurologic compromise. It can also detect spinal and paraspinal infections [19]. The MR images will reveal a T1 hypointense and T2 hyperintense mass in the epidural space (Figs. 18.1 and 18.2). Gadolinium enhancement increases sensitivity and gives a better differentiation between the abscess and the surrounding neurologic structures (Fig. 18.3).