Severe Medical Complications in Pregnancy | 19 |
Pregnant women with potentially serious medical illnesses are a challenge to the clinician. Women can develop new medical illnesses coincidental to pregnancy or may have underlying medical disease that worsens with the physiologic changes of pregnancy. Women are increasingly delaying pregnancy until older ages which are associated with more medical complications. An approach to evaluating the pregnant woman with potentially serious medical illness in the obstetric triage unit is outlined. Topics addressed include headache, shortness of breath (SOB) and pulmonary disease, chest pain, and cardiovascular disorders, as well as selected causes of abdominal pain.
HEADACHE
Headache is a common complaint in pregnancy and the majority of women presenting to obstetric triage have migraine, tension-type headaches, or headaches related to preeclampsia. Other serious and potential life-threatening causes of headaches that must be diagnosed and treated promptly are noted in Table 19.1.
Though most headaches caused by preeclampsia do not cause neurologic impairment or death, a subset of women with preeclampsia will have associated subarachnoid hemorrhage, intracerebral bleeding, or posterior reversible encephalopathy syndrome (PRES). The risk for subarachnoid hemorrhage from rupture of cerebral aneurysms and arteriovenous malformations is increased in pregnant women. The gravida is also at risk for cerebral vein thrombosis and benign increased intracranial hypertension (pseudotumor cerebri). A tumor can also present as a headache during pregnancy.
Migraine headaches often worsen in the beginning of pregnancy, paralleling the hormonal changes of the early pregnancy, and are described as headaches with characteristics similar to prepregnancy headaches. An aura, unilateral throbbing, or nausea and vomiting are all suggestive of a migraine headache. Triggering factors such as certain foods (nuts, aged cheeses, and caffeine), change in sleep pattern, or stress may be identified. Since many women eliminate caffeine intake with pregnancy, caffeine withdrawal may trigger headaches early in pregnancy. Tension-type headaches are typically tight, squeezing headaches that worsen later in the day and are not associated with aura, visual or other neurologic signs or symptoms, or nausea or vomiting. Warning signs that may suggest more serious causes of headache include sudden onset (thunderclap), new or different type of headache, worsening upon awakening in the morning or waking up at night, onset with exertion, association with neurologic impairment, or fever. Further investigations for patients with these concerning features may include CT of head, magnetic resonance imaging/angiography/venography (MRI/MRA/MRV) of the brain, and lumbar puncture (Mitsikostas et al., 2016; Skliut & Jamieson, 2016). In addition, victims of domestic violence may present with chronic complaints including headaches.
TABLE 19.1 Selected Causes of Headache in Pregnant Women Presenting to Obstetric Triage/Emergency Department
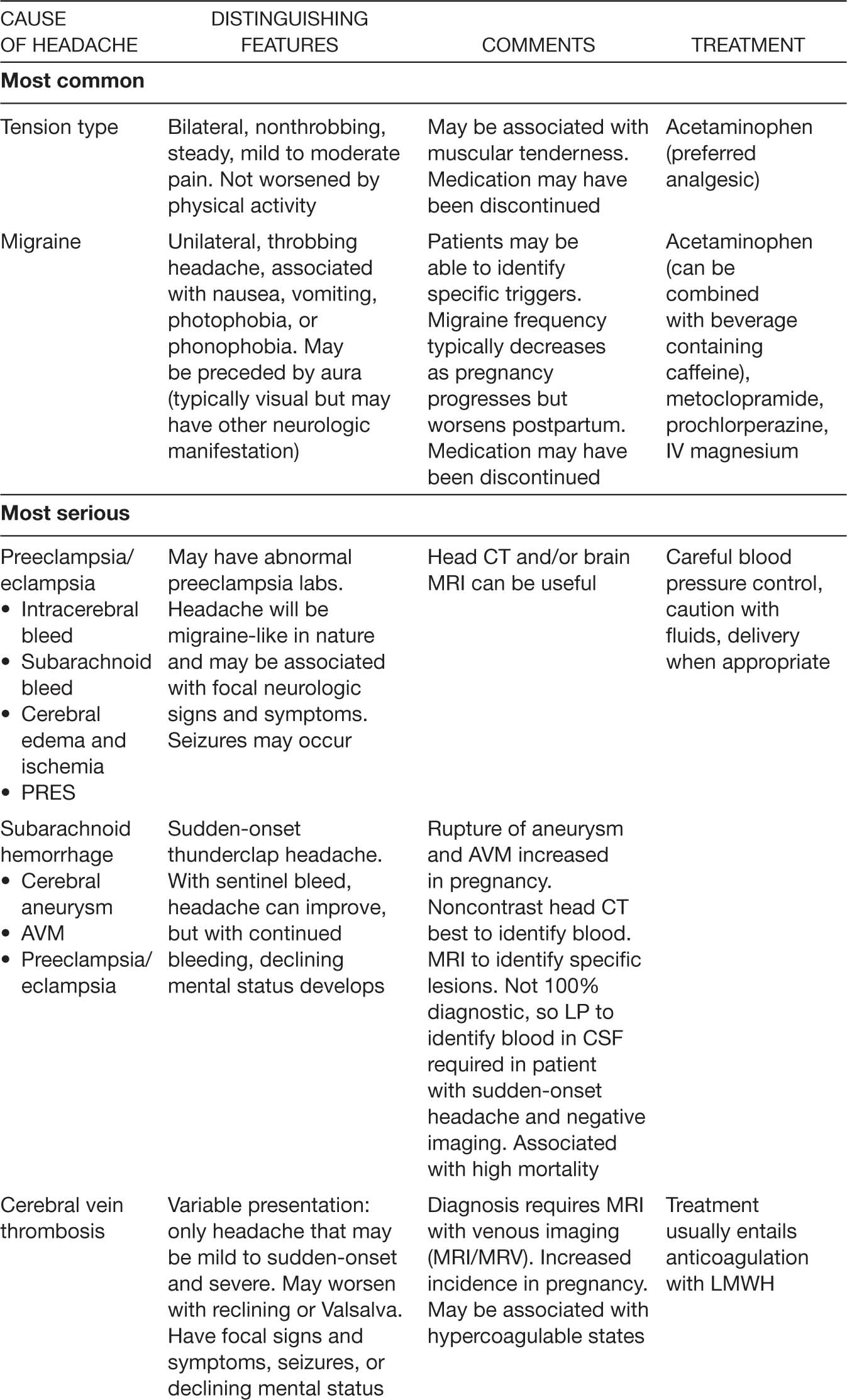
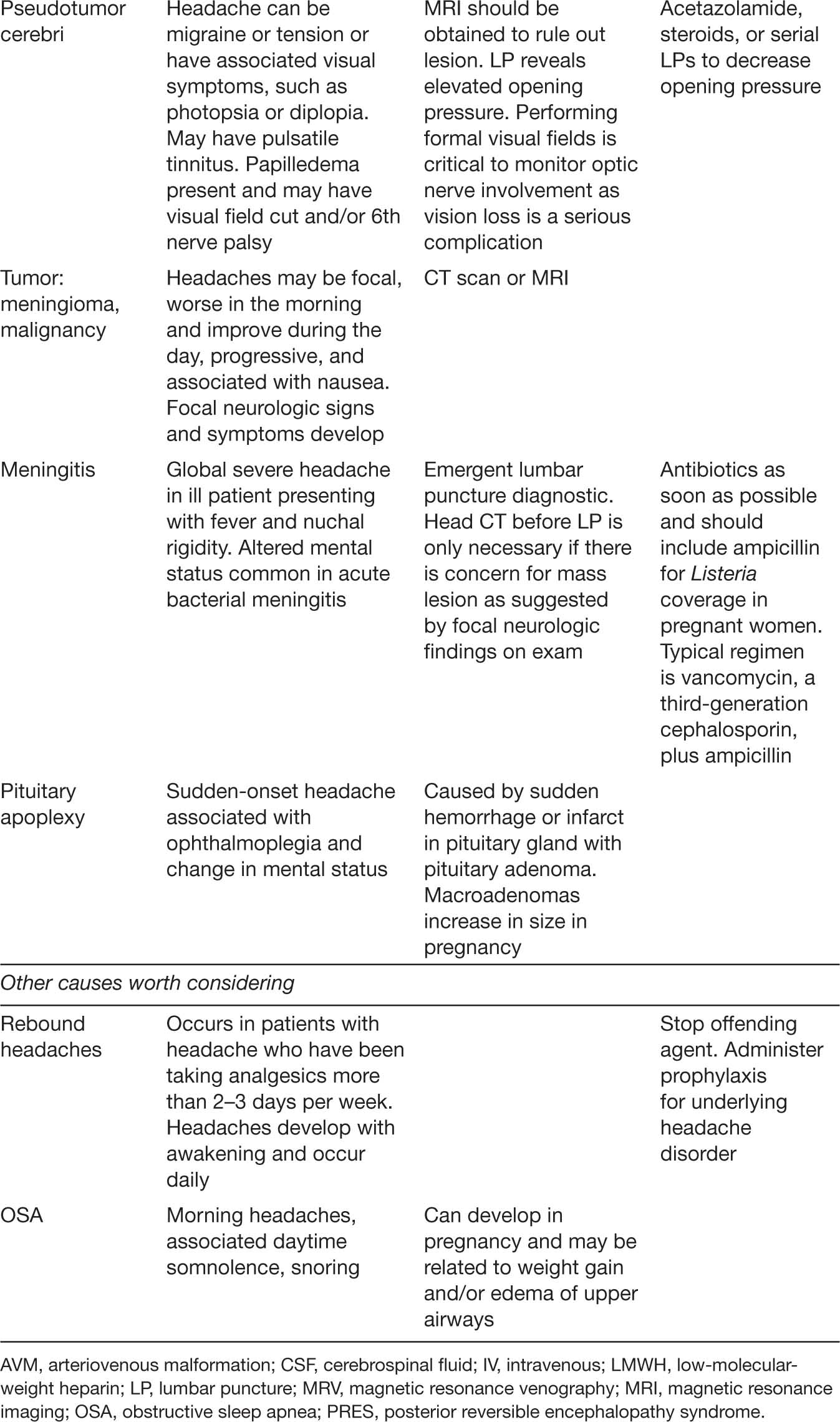
206When migraine or severe tension headache is not relieved by acetaminophen alone, the addition of caffeine (such as coffee or a cola drink) and/or metoclopramide or prochlorperazine may be helpful. Narcotics are frequently suboptimally effective and are associated with rebound headaches (Goadsby, Goldberg, & Silberstein, 2008), although they can be helpful in the acute setting. Intravenous (IV) magnesium (1–2 g) is useful for relief of acute migraines in nonpregnant women, and it is reasonable to assume it may be effective and safe during pregnancy. If acute treatment does not provide adequate control of headache, prophylactic medication such as riboflavin, beta-blockers, and tricyclic antidepressants are an option (R. E. Wells, Turner, Lee, Bishop, & Strauss, 2016).
RESPIRATORY ISSUES
Shortness of Breath
Dyspnea is a common complaint in normal pregnancy experienced by up to 50% of women. However, pathologic cardiopulmonary causes are essential to distinguish, as noted in Table 19.2.
The pregnant woman with dyspnea of pregnancy has symptoms at rest but not with exertion and may describe the need to “take deep breaths” or frequent “sighs.” They may also be SOB while talking on the telephone. These women are thought to be particularly sensitive to the normal increased tidal volume of pregnancy. Deconditioning or SOB associated with progressing pregnancy and weight gain may cause dyspnea on exertion, but the history and physical exam are otherwise reassuring. The presence of wheezing, chest tightness, cough, or nocturnal awakening suggests asthma as a cause of dyspnea.
A history of pulmonary or cardiac disease predating pregnancy suggests the possibility of worsening disease or an inability to tolerate pregnancy-related physiologic changes. Shortness of breath that presents at the peak of blood volume (28–32 weeks) may be due to underlying cardiac disease exacerbated by increased volume. Associated symptoms that suggest concerning underlying pathology include fever, cough, chest tightness or chest pain, orthopnea (though this can be present in normal pregnant women secondary to the elevated diaphragm), and paroxysmal nocturnal dyspnea (PND). Physical examination findings mandating a more extensive workup for underlying cardiopulmonary disease include increased respiratory rate, decreased pulse oximetry at rest or with exertion, tachycardia, abnormal lung exam, elevated jugular venous pulsation (JVP), and loud murmurs or gallops on cardiac exam. An initial workup includes a complete blood count (CBC), shielded anterior–posterior (AP) and lateral chest x-rays (CXR), and an EKG. Evaluation for pulmonary embolism (PE) with chest CT pulmonary angiogram (CTPA) and lower-extremity Doppler or ventilation perfusion scan (VQ scan), underlying structural heart abnormalities with an echocardiogram, or other lung pathology with pulmonary function tests may be indicated.
TABLE 19.2 Selected Causes of Shortness of Breath in Pregnant Women Presenting to Obstetric Triage/Emergency Department
ETIOLOGY | FEATURES | COMMENTS |
Dyspnea of pregnancy | Experience of “air hunger” or the need to take a deep breath. Patients note the need to “catch their breath” while talking on the phone. There are no symptoms or physical exam findings suggestive of underlying cardiac or pulmonary disease | Thought to be caused by awareness of the increased ventilation in pregnancy. Begins early in pregnancy, at an average of 18 weeks gestation. Tends to improve later in pregnancy |
Asthma | Complaints of chest tightness, dyspnea, and nocturnal awakening. Cough is common. May identify triggers such as cigarette smoke, gastroesophageal reflux, or sinusitis. Exam reveals wheezing | Pulmonary function tests and peak flow measurements useful. CXR is normal, if obtained. Treatment includes beta-agonists and steroids per the National Asthma Guideline Recommendations (National Asthma Education and Prevention Program, 2007) |
Pneumonia | Cough, fever, and SOB are typical presenting features. Physical exam reveals evidence of lung consolidation | CXR reveals pneumonia. Most gravidas with pneumonia require admission to monitor for progression and to ensure oxygen saturations remain above 95% |
PE | Variable presentation, which may include SOB, dizziness, sudden-onset pleuritic chest pain, hemoptysis, and palpitations. Exam may or may not reveal tachycardia, hypotension, hypoxia, pleural rub, or evidence of lower-extremity DVT | EKG may reveal sinus tachycardia of right heart strain but often normal. CXR may be normal. CTPA done in combination with lower-extremity Doppler or VQ scanning is diagnostic. D-dimers are -not validated in pregnancy. Treatment is anticoagulation with LMWH or unfractionated heparin. Patients may require IVC filter if near term, unable to tolerate anticoagulants, have failed anticoagulants, or have large clot burden |
Pulmonary edema | SOB associated with crackles on lung exam. Look for underlying disorder associated with pulmonary edema such as preeclampsia, sepsis or pyelonephritis, abnormal cardiac exam if associated underlying cardiac disease is present | May respond readily to diuresis with furosemide and treatment of underlying precipitating disorder. Physiologic changes that predispose to pulmonary edema include increased blood volume and lower oncotic pressure. With preeclampsia, there is associated endothelial damage and further lowering of oncotic pressure. If there is infection, there may be increased effects of endotoxin |
Pulmonary hypertension | Progressive SOB, which may or may not be associated with chest pain and syncope. Cardiac exam may reveal persistent S2 splitting, and there may be evidence of right-sided heart failure on exam in severe cases | EKG may reveal right heart strain. Echo may reveal elevated pulmonary artery pressures, but cardiac catheterization is necessary for accurate measurement. Search for secondary causes including PE is crucial. Pulmonary hypertension has a very high mortality in pregnancy |
Valvular heart disease | Progressive SOB with cough, orthopnea, PND, and exertional symptoms suggest cardiac disease. May have elevated JVP, cardiac murmur and gallop, crackles on lung exam, and lower-extremity edema | 208Cardiac echo is diagnostic. The physiologic demands of pregnancy may unmask previously well-compensated valvular heart disease |
Peripartum cardiomyopathy | In addition to progressive dyspnea, cough, orthopnea, and PND, patients may present with palpitations or syncope secondary to an arrhythmia. Exam reveals elevated JVP, displaced point of maximal impulse, gallop, crackles on lung exam, and edema | Cardiac echo reveals ventricular dysfunction. Presents later in pregnancy, and no other causes of cardiomyopathy are identified. Preeclampsia must be differentiated. Causes of death include arrhythmia, thromboembolic disease, and progressive heart failure |
MI | Classic presentation includes substernal chest tightness that radiates to left shoulder/arm and jaw and is associated with SOB, nausea, vomiting, and diaphoresis. Atypical presentations are common in women, so high index of suspicion is necessary | EKG and cardiac enzymes are used for diagnosis. Women may not have traditional risk factors such as diabetes, hypertension, hyperlipidemia, and smoking. Mechanism may be coronary artery dissection, thrombosis in a normal coronary artery, or vasospasm, in addition to coronary artery disease. Cocaine should be considered |
CTPA, computerized tomography pulmonary arteriogram; CXR, chest radiograph; DVT, deep venous thrombosis; EKG, electrocardiogram; IVC, inferior vena cava; JVP, jugular venous pressure; LMWH, low-molecular-weight heparin; MI, myocardial ischemia/infarct; PE, pulmonary embolism; PND, paroxysmal nocturnal dyspnea; SOB, shortness of breath; VQ, ventilation perfusion scan.
Pulmonary Embolism
PE in pregnancy is a leading cause of maternal death in developed nations (Zeitlin & Mohangoo, 2008). Factors contributing to the increased risk of thrombosis in pregnancy include venodilation secondary to hormonal and mechanical factors, increase in prothrombotic and decrease in fibrinolytic factors, and venous trauma at labor and delivery. Risk factors for thrombosis in pregnancy include history of thrombophilia, smoking, elevated body mass index, antepartum immobilization, age, parity, cesarean delivery, preeclampsia, and assisted reproductive techniques (Jacobson, Skejeldestad, & Sandset, 2008; James, Jamison, Brancazio, & Myers, 2006; van Walraven et al., 2003).
There is no one clinical sign or symptom that is consistently seen in gravidas with PE, and a high index of suspicion is needed to prevent missing this potentially fatal diagnosis. Women may or may not present with chest pain, SOB, tachypnea, tachycardia, hypoxia, or abnormal chest x-ray or EKG. In one study, over half of the pregnant women with documented PE had normal pO2 on arterial blood gas and normal A–a gradients (Powrie et al., 1998). In the nonpregnant population, the Wells criteria (P. S. Wells et al., 2000) and Geneva criteria (Le Gal et al., 2006) are useful clinical decision tools for determining 209the probability of PE, but elements of these tools, such as heart rate, are altered by pregnancy physiology. Furthermore, the use of d-dimers is hampered by increasing levels as gestation progresses and cannot be considered reliable to rule out PE in the gravida without further studies (Konkle, 2015).
Because these tools have not been validated in pregnancy, diagnostic imaging is the cornerstone for the diagnosis of PE in pregnant women. The initial evaluation includes a CXR that may reveal an alternate diagnosis with minimal radiation exposure. PE can then be definitively diagnosed with either a VQ scan or CTPA. An advantage of the CTPA is the potential to identify an alternate diagnosis with lower fetal radiation exposure. However, the risk for maternal breast cancer may be increased because CTPA exposes the maternal breasts to as much as 2 to 5 rads of radiation (Miller, Chalhoub, & Bourjeily, 2011). Some centers use breast shields to mitigate this risk. In addition, CTPA is more likely to be technically limited in pregnancy because the increased blood volume and cardiac output affect the arrival of contrast to the pulmonary artery. The VQ scan has a strong negative predictive value for PE, and fetal radiation exposure is still well within the acceptable range. Some clinicians prefer it to CTPA, particularly for women who have had previous CT scans or who are otherwise at increased risk for breast cancer. Local expertise in interpreting the results of VQ or CTPA is also a driving force in determining the best test to order. In the pregnant woman with symptoms of PE and findings suggestive of a lower-extremity deep vein thrombosis (DVT), a lower-extremity Doppler ultrasound is a reasonable first test. If a DVT is identified, PE can be presumed and therapeutic anticoagulation is indicated regardless.
The mortality of untreated PE is 30%, and death can occur from a recurrent PE within several hours of the initial event. Since anticoagulation decreases mortality to 2% to 8%, it is crucial to begin treatment quickly (Kearon et al., 2008). In women with a high suspicion for PE and low risk for bleeding, anticoagulation is begun immediately so as not to delay for the results of testing. Stable women with a potential alternative diagnosis in whom testing is performed quickly can be treated after the results of investigations are known. The preferred initial treatment for PE is low-molecular-weight heparin (LMWH) because of its proven mortality benefits, associated decreased recurrence of thrombosis, better bioavailability, ease of administration, and decreased risk of heparin-induced thrombocytopenia (Greer, 2015). Intravenous unfractionated heparin still has an important role for use in women at high risk for bleeding; near delivery; with significant hypotension, obesity, or renal insufficiency; or in whom thrombolytics may be considered.
Although the use of thrombolytics has been reported in pregnancy with a risk of bleeding similar to that of nonpregnant women, thrombolytics are most likely to be beneficial in the hemodynamically unstable gravida with refractory hypoxemia (Leonhardt, Gaul, Nietsch, Buerke, & Schleussner, 2006). Inferior vena cava (IVC) filters may be considered for use in pregnant women with PE who have contraindications to anticoagulation or in whom a large clot burden has been identified in the lower extremities or pelvis, which could potentially cause a fatal recurrent PE (Harris, Velineni, & Davies, 2016).
Asthma
Asthma is a common medical condition in pregnancy, affecting approximately 3.7% to 8.4% of pregnancies in the United States (Kwon, Belanger, & Bracken, 2003). Though asthma does not necessarily worsen in pregnancy, approximately one third of women will develop asthma exacerbations during gestation. 210Exacerbations are more likely to occur in women who have more severe asthma before pregnancy (Schatz et al., 2003). Factors specific to pregnancy that may contribute to exacerbations include hormonal changes, noncompliance with medications, increased gastroesophageal reflux, and possible triggering by rhinitis of pregnancy.
Gravidas with an asthma exacerbation may present with SOB, wheezing, cough, and/or chest tightness. Physical exam may reveal increased respiratory and heart rate, hypoxia, wheezing, and decreased air flow. Use of accessory muscles of respiration and paradoxical breathing portend respiratory failure. Peak flows are helpful to determine severity of exacerbation if performed with proper technique. Though pulse oximetry determines oxygenation, an arterial blood gas is necessary in women suspected of having more serious exacerbations to assess maternal PaCO2. In pregnancy, the normal maternal PaO2 is 100 to 105 mmHg and average PaCO2 is 30 mmHg. For fetal well-being, it is necessary to maintain the pregnant woman’s oxygenation saturation greater than 95% or the maternal pO2 at not less than 70 mmHg of oxygen. The pregnant asthmatic with a PaCO2 of 35 mmHg is already retaining CO2 and signifies impending respiratory failure. Because of the increased rates of aspiration and failed intubation in pregnancy, it is critical to anticipate the potential need for intubation in advance so that equipment and experienced personnel are prepared.
Supplemental oxygen, short-acting beta-agonists (SABA), and steroids are used for treatment of acute asthma exacerbations in pregnant women as they are in the nonpregnant population (Dombrowski et al., 2008; National Asthma Education and Prevention Program, 2007). Albuterol can be administered by nebulizer or metered dose inhaler and is initially given every 20 minutes for three doses followed by hourly doses as needed. Women who do not respond quickly to SABA or who are already taking steroids require the addition of methylprednisolone or prednisone. Ipratropium is added for severe exacerbations. Chest radiograph is indicated if there is concern about an underlying pulmonary process such as pneumonia. Women can be discharged with close outpatient follow-up if the peak expiratory flow rate is greater than or equal to 70% of predicted, there is a sustained response 60 minutes after the last treatment, no supplemental oxygen is required to keep oxygen saturation greater than 95% at rest or with exertion, there is no distress, and physical exam is normal. A low threshold for admission in pregnancy is prudent if there are any concerning symptoms because pregnant women have less respiratory reserve and higher oxygen saturation requirements than do nonpregnant women.
CARDIAC AND VASCULAR ISSUES
Chest Pain
The causes of chest pain in pregnancy range from benign and self-limiting to potentially life threatening. An initial history and physical exam help to narrow the differential. Often, the clinician is able to tell patients what the pain “isn’t” with more certainty than what the pain “is.” It can be reassuring that once the life-threatening causes are eliminated, it is unlikely that the cause of the chest pain will be harmful to either the pregnant woman or fetus and will likely resolve quickly. Many causes of chest pain are also causes of SOB, and the evaluation for these disorders overlap. The most serious causes of chest pain that must not be missed include PE, myocardial ischemia, and aortic dissection. Table 19.3 provides an overview of key points for chest pain in pregnancy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








