Chapter 20
Renal Replacement Therapy
Acute kidney injury (AKI) commonly occurs in intensive care unit (ICU) patients. Despite advances in both critical care therapy and renal replacement therapy (RRT), mortality continues to exceed 50% in ICU patients with AKI requiring RRT. When patients develop AKI, the ICU team and consulting nephrologist should collaborate to (1) reverse or prevent progression of AKI (see Chapter 82), (2) initiate renal replacement therapy at an optimal time, (3) choose the RRT modality that best suits the patient’s particular circumstances, (4) decide on the dose of solute clearance, and (5) select the ultrafiltration goal. This chapter reviews the thresholds for initiating RRT, the pros and cons of available RRT modalities, the optimal dose of solute clearance, and how to determine the ultrafiltration goal.
When to Start Renal Replacement Therapy
Most authorities would agree that certain signs and symptoms are strong indications for commencing RRT (Box 20.1). The value of early dialysis in asymptomatic patients, however, remains unproved. Some, but not all, uncontrolled and retrospective studies suggest that prophylactic dialysis aimed at maintaining the blood urea nitrogen (BUN) below 80 to 100 mg/dL (28 to 35 mmol/L) reduced mortality and morbidity in patients with AKI. Even two studies using concurrent controls to examine this issue provide contradictory results. The optimal timing for initiation of RRT in asymptomatic patients with AKI will require an adequately powered prospective randomized trial. However, the adequate design of such a trial is limited by the current inability to quickly and prospectively identify patients with early AKI who will have protracted renal injury and eventually require RRT.
Physicians should exercise caution when using a BUN of 100 mg/dL as a threshold for initiating RRT. BUN can be raised out of proportion to the serum creatinine level, secondary to excessive protein administration, gastrointestinal bleeding, or tetracycline or corticosteroid use. Patients may exhibit a BUN exceeding the 100 mg/dL threshold but lack evidence of uremia or the need for dialysis. Alternatively, in patients with decreased urea generation caused by poor nutrition or liver disease, manifestations of the uremic syndrome may appear despite a BUN below this threshold.
Available Modalities
A thorough knowledge of the efficacy, advantages, disadvantages, route of access, and cost of each modality is critical when choosing the optimal renal replacement method (Table 20.1). There are three types of RRT therapies: intermittent therapy, continuous therapy, and hybrid therapy. In terms of efficacy, the major considerations are efficiency of solute clearance, volume removal (ultrafiltration), and the impact on patient survival.
TABLE 20.1
Characteristics of Renal Replacement Modalities
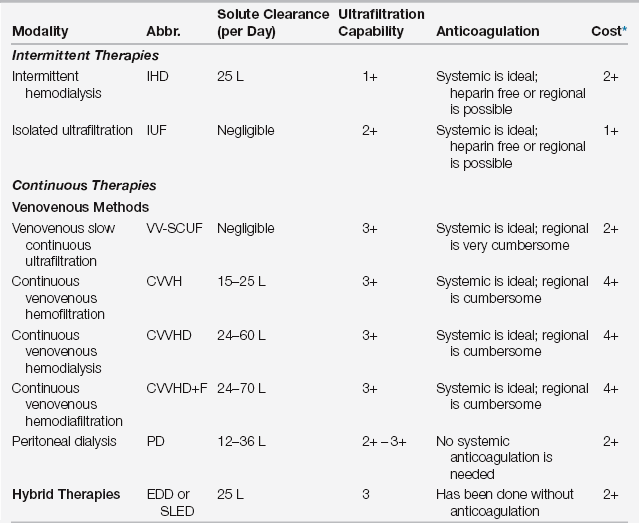
∗Cost refers to expenses accrued by the dialysis cost center and does not include extra work by ICU house staff or nursing staff (which would be highest for the continuous venovenous modalities).
Intermittent Therapies
Intermittent Hemodialysis
The cardiovascular defense mechanisms that normally maintain blood pressure during fluid removal are often impaired, overwhelmed, or both in critically ill patients. Such patients frequently exhibit cardiac dysfunction or peripheral vasodilation, for example, secondary to sepsis or liver failure. This hypotension may have many sequelae, including delayed recovery of AKI and ischemia to many organs, including the heart and intestine. Another major disadvantage of IHD is the requirement for a specialized dialysis nurse to devote individual (one-on-one) care to the critically ill patient at the ICU bedside, as opposed to caring for three or four more stable patients in a dialysis unit.
Isolated Ultrafiltration
Isolated ultrafiltration (IUF) resembles IHD and also uses a double-lumen central venous catheter for access. The major difference between IUF and IHD is that dialysate is not pumped through the filter. The pressure settings across the dialyzer are programmed to remove a certain fluid volume. IUF is indicated in fluid-overloaded patients, resistant to diuretics and without significant accumulation of nitrogenous wastes, hyperkalemia, or metabolic acidosis (as solute removal is negligible). IUF is less likely than conventional IHD to induce intradialytic hypotension. Simultaneous ultrafiltration and solute removal as occurs during standard IHD result in a rapid decrease in intravascular osmolality, which reduces the rate of osmotically induced plasma refilling from the intracellular and interstitial compartments. In contrast, intravascular osmolality remains stable with IUF, the rate of plasma refilling is relatively rapid, and blood pressure is better maintained than during IHD. Another mechanism that contributes to the better hemodynamic stability during IUF, as compared to IHD, is that IUF results in pronounced energy loss from the patient to the extracorporeal circuit. This energy loss results in a lower patient core temperature, which in turn increases peripheral vascular resistance and therefore blood pressure.
Continuous Therapies
Continuous renal replacement therapy (CRRT) was historically delivered via an arteriovenous circuit. However, high rates of complications (arterial thrombosis and bleeding) occurred, and the limited blood flow in critically ill hypotensive patients resulted in frequent filter clotting and reduced solute clearance. These problems led to obsolescence of the arteriovenous approach, now virtually entirely replaced by a venovenous approach. Using a single double-lumen central venous catheter for access, the venovenous modalities require a blood pump to provide a constant blood flow independent of the mean arterial blood pressure. Venovenous CRRT machines are similar to IHD machines, with air-leak detectors, pressure monitors, and alarms in the circuit. Variation in the amount of ultrafiltration and use of dialysate allows for four different variations of the procedure (see Table 20.1). The major advantage of continuous as compared to intermittent therapies is that fluid may be removed continuously over 24 hours, creating less stress in hemodynamically compromised patients. There is also less variation in electrolyte and acid base parameters, as serum potassium levels tend to rise and serum bicarbonate tends to decrease during the interdialytic interval of IHD.
Venovenous Slow Continuous Ultrafiltration (VV-SCUF)
The equipment required for VV-SCUF is shown in Figure 20.1. Blood circulates from the “arterial lumen” of a double-lumen catheter placed in a central vein through a filter and returns to the patient through the “venous” lumen of the same double-lumen catheter. No diffusive dialysis takes place because no dialysis solution is used. The limitations are similar to IUF.
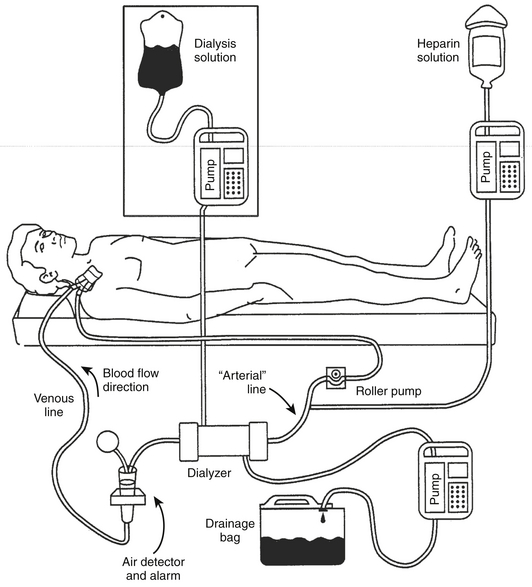
Figure 20.1 The equipment and circuit required for venovenous slow continuous ultrafiltration (VV-SCUF).
The additional equipment required to perform continuous venovenous hemodialysis (CVVHD) is shown in the boxed area. The “arterial” line also normally has a pressure transducer in-line (omitted from figure) between the roller pump and the dialyzer to monitor perfusion pressure. (Modified from Daugirdas JT, Ing TS: Handbook of Dialysis. Boston: Little, Brown, 1988.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








