Key Concepts
 The combined blood flow through both kidneys normally accounts for 20-25% of total cardiac output.
The combined blood flow through both kidneys normally accounts for 20-25% of total cardiac output.
 Autoregulation of renal blood flow normally occurs between mean arterial blood pressures of 80 and 180 mm Hg and is principally due to intrinsic myogenic responses of the afferent glomerular arterioles to blood pressure changes.
Autoregulation of renal blood flow normally occurs between mean arterial blood pressures of 80 and 180 mm Hg and is principally due to intrinsic myogenic responses of the afferent glomerular arterioles to blood pressure changes.
 Renal synthesis of vasodilating prostaglandins (PGD2, PGE2, and PGI2) is an important protective mechanism during periods of systemic hypotension and renal ischemia.
Renal synthesis of vasodilating prostaglandins (PGD2, PGE2, and PGI2) is an important protective mechanism during periods of systemic hypotension and renal ischemia.
 Dopamine and fenoldopam dilate afferent and efferent arterioles via D1-receptor activation. Fenoldopam and low-dose dopamine infusion can at least partially reverse norepinephrine-induced renal vasoconstriction.
Dopamine and fenoldopam dilate afferent and efferent arterioles via D1-receptor activation. Fenoldopam and low-dose dopamine infusion can at least partially reverse norepinephrine-induced renal vasoconstriction.
 Reversible decreases in renal blood flow, glomerular filtration rate, urinary flow, and sodium excretion occur during both regional and general anesthesia. Acute kidney injury is less likely if an adequate intravascular volume and a normal blood pressure are maintained.
Reversible decreases in renal blood flow, glomerular filtration rate, urinary flow, and sodium excretion occur during both regional and general anesthesia. Acute kidney injury is less likely if an adequate intravascular volume and a normal blood pressure are maintained.
 The endocrine response to surgery and anesthesia is at least partly responsible for transient fluid retention seen postoperatively in many patients.
The endocrine response to surgery and anesthesia is at least partly responsible for transient fluid retention seen postoperatively in many patients.
 Compound A, a breakdown product of sevoflurane, has been shown to cause renal damage in laboratory animals. Its accumulation in the breathing circuit is favored by low flow rates. No clinical study has detected significant renal injury in humans during sevoflurane anesthesia; nonetheless, some regulatory authorities recommend fresh gas flow of at least 2 L/min with sevoflurane to prevent this theoretical problem.
Compound A, a breakdown product of sevoflurane, has been shown to cause renal damage in laboratory animals. Its accumulation in the breathing circuit is favored by low flow rates. No clinical study has detected significant renal injury in humans during sevoflurane anesthesia; nonetheless, some regulatory authorities recommend fresh gas flow of at least 2 L/min with sevoflurane to prevent this theoretical problem.
 The pneumoperitoneum produced during laparoscopy causes an abdominal compartment syndrome-like state. The increase in intraabdominal pressure typically produces oliguria (or anuria) that is generally proportional to the insufflation pressures. Mechanisms include central venous compression (renal vein and vena cava); renal parenchymal compression; decreased cardiac output; and increases in plasma levels of renin, aldosterone, and antidiuretic hormone.
The pneumoperitoneum produced during laparoscopy causes an abdominal compartment syndrome-like state. The increase in intraabdominal pressure typically produces oliguria (or anuria) that is generally proportional to the insufflation pressures. Mechanisms include central venous compression (renal vein and vena cava); renal parenchymal compression; decreased cardiac output; and increases in plasma levels of renin, aldosterone, and antidiuretic hormone.
Renal Physiology & Anesthesia: Introduction
The kidneys play a vital role in regulating the volume and composition of body fluids, eliminating toxins, and elaborating hormones, including renin, erythropoietin, and the active form of vitamin D. Factors directly and indirectly related to operative procedures and to anesthetic management frequently have a physiologically significant impact on renal physiology and renal function, and may lead to perioperative fluid overload, hypovolemia, renal insufficiency, and kidney failure, which are major causes of perioperative morbidity and mortality.
Diuretics are frequently used in the perioperative period. Diuretics are commonly administered on a chronic basis to patients with cardiovascular disease, including hypertension and chronic heart failure, and to patients with liver and kidney disease. Diuretics may be used intraoperatively, particularly during neurosurgical, cardiac, major vascular, ophthalmic, and urological procedures. Familiarity with the various types of diuretics, their mechanisms of action, side effects, and potential anesthetic interactions, is therefore essential.
The Nephron
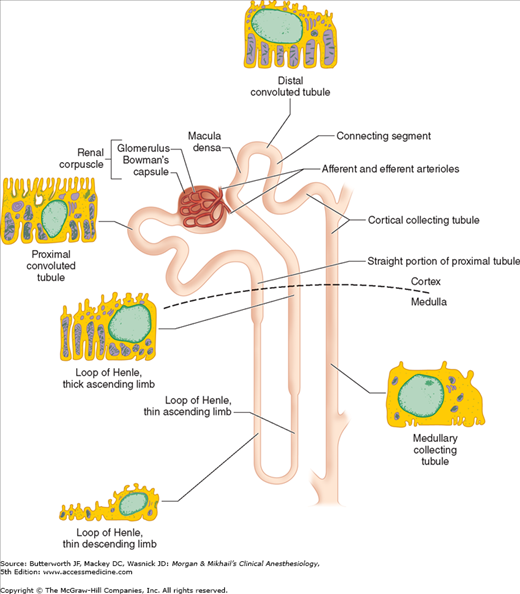
Each kidney is made up of approximately 1 million functional units called nephrons. Anatomically, a nephron consists of a tortuous tubule with at least six specialized segments. At its proximal end (the renal corpuscle, composed of a glomerulus and a Bowman’s capsule), an ultrafiltrate of blood is formed, and as this fluid passes through the nephron, its volume and composition are modified by both the reabsorption and the secretion of solutes. The final product is eliminated as urine.
Nephrons are classified as cortical or juxtamedullary (see below), and the renal corpuscles of all nephrons are located in the renal cortex. The six major anatomical and functional divisions of the nephron are the renal corpuscle, the proximal convoluted tubule, the loop of Henle, the distal renal tubule, the collecting tubule, and the juxtaglomerular apparatus (Figure 29-1 and Table 29-1).
| Segment | Function |
|---|---|
| Renal corpuscle (glomerulus, Bowman’s capsule) | Ultrafiltration of blood |
| Proximal tubule |
|
| Loop of Henle |
|
| Distal tubule |
|
| Collecting tubule |
|
| Juxtaglomerular apparatus | Secretion of renin |
Each renal corpuscle contains a glomerulus, which is composed of tufts of capillaries that jut into Bowman’s capsule, providing a large surface area for the filtration of blood. Blood flow is provided by a single afferent arteriole and is drained by a single efferent arteriole (see below). Endothelial cells of the glomeruli are separated from the epithelial cells of Bowman’s capsule only by their fused basement membranes. The endothelial cells are perforated with relatively large fenestrae (70-100 nm), but the epithelial cells interdigitate tightly with one another, leaving relatively small filtration slits (about 25 nm). The two cell types with their basement membranes provide an effective filtration barrier to cells and large-molecular-weight substances. This barrier has multiple anionic sites that give it a net negative charge, favoring filtration of cations relative to anions. A third cell type, called intraglomerular mesangial cells, is located between the basement membrane and epithelial cells near adjacent capillaries. These contractile cells regulate glomerular blood flow and also exhibit phagocytic activity. They secrete various substances, absorb immune complexes, and contain contractile proteins that respond to vasoactive substance. Mesangial cells contract, reducing glomerular filtration, in response to angiotensin II, vasopressin, norepinephrine, histamine, endothelins, thromboxane A2, leukotrienes (C4 and D4), prostaglandin F2, and platelet-activating factor. They relax, thereby increasing glomerular filtration, in response to atrial natriuretic peptide (ANP), prostaglandin E2, and dopaminergic agonists.
Glomerular filtration pressure (about 60 mm Hg) is normally approximately 60% of mean arterial pressure and is opposed by both plasma oncotic pressure (about 25 mm Hg) and renal interstitial pressure (about 10 mm Hg). Afferent and efferent arteriolar tone are both important in determining glomerular filtration pressure: filtration pressure is directly proportional to efferent arteriolar tone but inversely proportional to afferent tone. Approximately 20% of plasma is normally filtered as blood passes through the glomerulus.
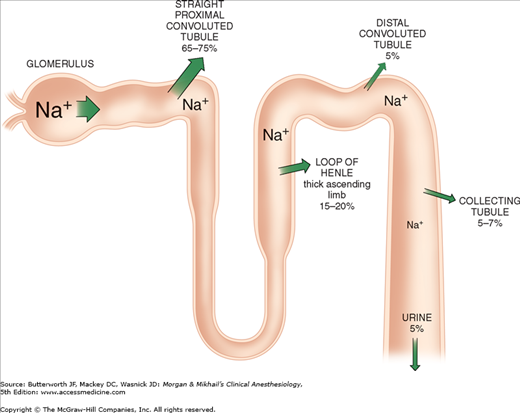
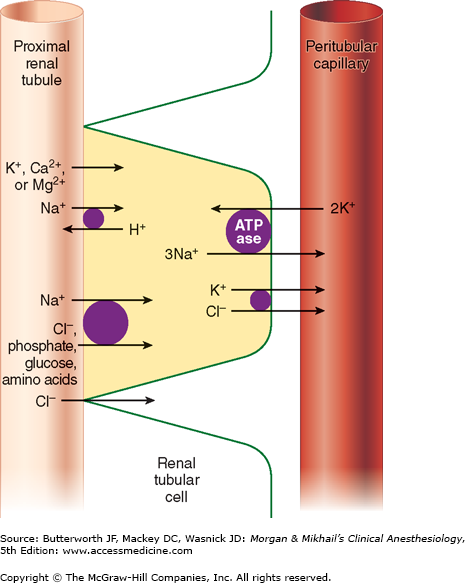
Of the ultrafiltrate formed in Bowman’s capsule 65-75% is normally reabsorbed isotonically (proportional amounts of water and sodium) in the proximal renal tubules (Figure 29-2). To be reabsorbed, most substances must first traverse the tubular (apical) side of the cell membrane, and then cross the basolateral cell membrane into the renal interstitium before entering peritubular capillaries. The major function of the proximal tubule is Na+ reabsorption. Sodium is actively transported out of proximal tubular cells at their capillary side by membrane-bound Na+-K+–adenosine triphosphatase (Na+-K+-ATPase) (Figure 29-3). The resulting low intracellular concentration of Na+allows passive movement of Na+ down its gradient from tubular fluid into epithelial cells. Angiotensin II and norepinephrine enhance Na+ reabsorption in the early proximal tubule. In contrast, dopamine and fenoldopam decrease the proximal reabsorption of sodium via D1-receptor activation.
Sodium reabsorption is coupled with the reabsorption of other solutes and the secretion of H+ (Figure 29-3). Specific carrier proteins use the low concentration of Na+ inside cells to transport phosphate, glucose, and amino acids. The net loss of intracellular positive charges, the result of Na+-K+-ATPase activity (exchanging 3Na+ for 2K+), favors the absorption of other cations (K+, Ca2+, and Mg2+). Thus, the Na+-K+-ATPase at the basolateral side of the renal cells provides the energy for the reabsorption of most solutes. Sodium reabsorption at the luminal membrane is also coupled with countertransport (secretion) of H+. The latter mechanism is responsible for reabsorption of 90% of the filtered bicarbonate ions (see Figure 50-3). Unlike other solutes, chloride can traverse the tight junctions between adjacent tubular epithelial cells, and accordingly, is passively resorbed via its concentration gradient. Active chloride reabsorption may also take place as a result of a K+-Cl– cotransporter that extrudes both ions at the capillary side of the cell membrane (Figure 29-3). Water moves passively out the proximal tubule along osmotic gradients. Apical membranes of epithelial cells contain specialized water channels, composed of a membrane protein called aquaporin-1, that facilitate water movement.
The proximal tubules are capable of secreting organic cations and anions. Organic cations such as creatinine, cimetidine, and quinidine may share the same pump mechanism and thus can compete for excretion with one another. Organic anions such as urate, ketoacids, penicillins, cephalosporins, diuretics, salicylates, and most radiocontrast dyes also share common secretory mechanisms. Both pumps probably play a major role in the elimination of many circulating toxins. Low-molecular-weight proteins, which are filtered by glomeruli, are normally reabsorbed by proximal tubular cells, to be metabolized intracellularly.
The loop of Henle consists of descending and ascending portions. They are responsible for maintaining a hypertonic medullary interstitium and also indirectly provide the collecting tubules with the ability to concentrate urine. The thin descending segment is a continuation of the proximal tubule and descends from the renal cortex into the renal medulla. In the medulla, the descending portion acutely turns back upon itself and rises back up toward the cortex as the ascending portion. The ascending portion consists of a functionally distinct, thin ascending limb, a medullary thick ascending limb, and a cortical thick ascending limb (Figure 29-1). Cortical nephrons have relatively short loops of Henle which extend only into the more superficial regions of the renal medulla and often lack a thin ascending limb. Juxtamedullary nephrons, which have renal corpuscles located near the renal medulla, possess loops of Henle that project deeply into the renal medulla. Cortical nephrons outnumber juxtamedullary nephrons by approximately 7:1.
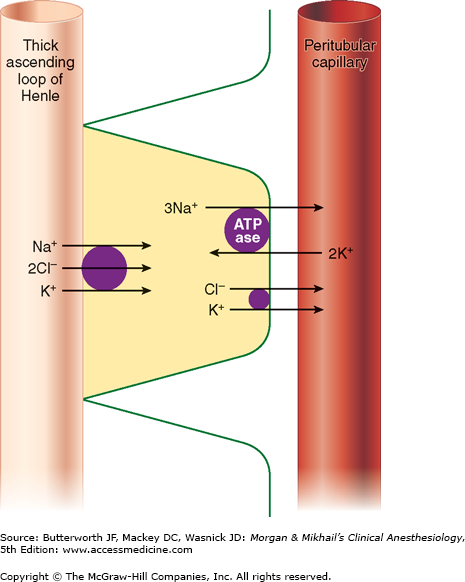
Only 25-35% of the ultrafiltrate formed in Bowman’s capsule normally reaches the loop of Henle. Once there, 15-20% of the filtered sodium load is normally reabsorbed in the loop of Henle. With the notable exception of the ascending thick segments, solute and water reabsorption in the loop of Henle is passive and follows concentration and osmotic gradients, respectively. In the ascending thick segment, however, Na+ and Cl− are reabsorbed in excess of water; moreover, Na+ reabsorption in this part of the nephron is directly coupled to both K+ and Cl− reabsorption (Figure 29-4), and [Cl−] in tubular fluid appears to be the rate-limiting factor. Active Na+ reabsorption still results from Na+-K+-ATPase activity on the capillary side of epithelial cells.
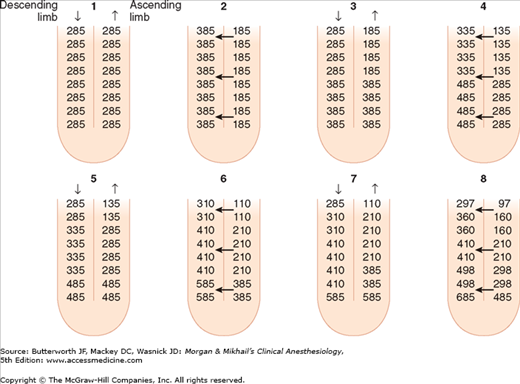
Unlike the descending limb and the thin ascending limb, the thick parts of the ascending limb are impermeable to water. As a result, tubular fluid flowing out of the loop of Henle is hypotonic (100-200 mOsm/L) and the interstitium surrounding the loop of Henle is therefore hypertonic. A countercurrent multiplier mechanism is established such that both the tubular fluid and medullary interstitium become increasingly hypertonic with increasing depth into the medulla (Figure 29-5). Urea concentrations also increase within the medulla and contribute to the hypertonicity. The countercurrent mechanism includes the loop of Henle, the cortical and medullary collecting tubules, and their respective capillaries (vasarecta).
Figure 29-5
The countercurrent multiplier mechanism. This mechanism is dependent on differential permeability and transport characteristics between the descending and ascending limbs. The descending limb and the thin ascending limb are permeable to water, Na+, Cl−, and urea. The thick ascending limb is impermeable to water and urea, actively reabsorbs Na+ and Cl–, and therefore can generate an osmotic gradient. This figure depicts from “time zero,” a progressive 200-mOsm/kg gradient between the descending and ascending limbs. Note that as urine flows, the gradient remains unchanged but the osmolality progressively increases at the bottom of the loop. (Reproduced, with permission, from Pitts RF: Physiology of the Kidney and Body Fluids, 3rd ed. Year Book, 1974.)
The thick ascending loop of Henle is also an important site for calcium and magnesium reabsorption, and parathyroid hormone may augment calcium reabsorption at this location.
The distal tubule receives hypotonic fluid from the loop of Henle and is normally responsible for only minor modifications of tubular fluid. In contrast to more proximal portions, the distal nephron has very tight junctions between tubular cells and is relatively impermeable to water and sodium. It can therefore maintain the gradients generated by the loop of Henle. Sodium reabsorption in the distal tubule normally accounts for only about 5% of the filtered sodium load. As in other parts of the nephron, the energy is derived from Na+-K+-ATPase activity on the capillary side, but on the luminal side Na+ is reabsorbed by an Na+-Cl− carrier. Sodium reabsorption in this segment is directly proportional to Na+ delivery. The distal tubule is the major site of parathyroid hormone-and vitamin D-mediated calcium reabsorption.
The latter portion of the distal tubule is referred to as the connecting segment. Although it is also involved in hormone-mediated calcium reabsorption, unlike more proximal portions, it participates in aldosterone-mediated Na+ reabsorption.
The collecting tubule can be divided into cortical and medullary portions. Together, they normally account for the reabsorption of 5-7% of the filtered sodium load.
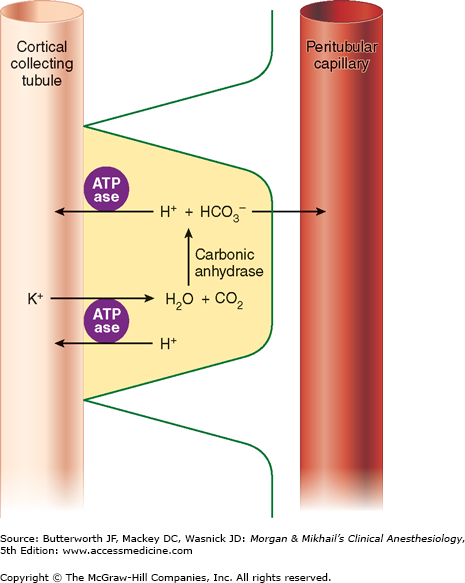
This part of the nephron consists of two cell types: (1) principal cells (P cells), which primarily secrete potassium and participate in aldosterone-stimulated Na+ reabsorption, and (2) intercalated cells (I cells), which are responsible for acid-base regulation. Because P cells reabsorb Na+ via an electrogenic pump, either Cl− must also be reabsorbed or K+ must be secreted to maintain electroneutrality. Increased intracellular [K+] favors K+ secretion. Aldosterone enhances Na+-K+-ATPase activity in this part of the nephron by increasing the number of open K+ and Na+ channels in the luminal membrane. Aldosterone also enhances the H+-secreting ATPase on the luminal border of I cells (Figure 29-6). I cells additionally have a luminal K+-H+-ATPase pump, which reabsorbs K+ and secretes H+, and some I cells are capable of secreting bicarbonate ion in response to large alkaline loads.
The medullary collecting tubule courses down from the cortex through the hypertonic medulla before joining collecting tubules from other nephrons to form a single ureter in each kidney. This part of the collecting tubule is the principal site of action for antidiuretic hormone (ADH), also called arginine vasopressin (AVP). ADH stimulates the expression of a water channel protein, aquaporin-2, in the cell membrane. The permeability of the luminal membrane to water is entirely dependent on the presence of ADH (see Chapter 49). Dehydration increases ADH secretion, rendering the luminal membrane permeable to water. As a result, water is osmotically drawn out of the tubular fluid passing through the medulla, resulting in production of concentrated urine (up to 1400 mOsm/L). Conversely, adequate hydration suppresses ADH secretion, allowing fluid in the collecting tubules to pass through the medulla relatively unchanged and to remain hypotonic (100-200 mOsm/L). This part of the nephron is responsible for acidifying urine; the hydrogen ions secreted are excreted in the form of titratable acids (phosphates) and ammonium ions (see Chapter 50).
Differences in permeability to urea in the cortical and medullary collecting tubules account for up to half the hypertonicity of the renal medulla. Cortical collecting tubules are freely permeable to urea, whereas medullary collecting tubules are normally impermeable. In the presence of ADH, the innermost part of the medullary collecting tubules becomes even more permeable to urea. Thus, when ADH is secreted, water moves out of the collecting tubules and the urea becomes highly concentrated. Urea can then diffuse out deeply into the medullary interstitium, increasing its tonicity.
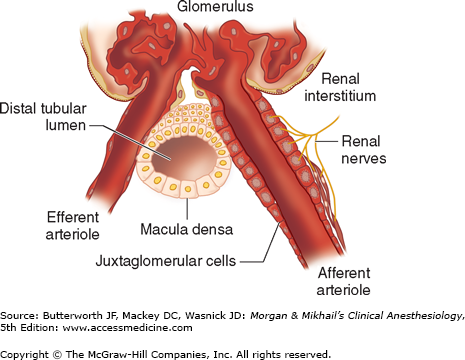
This small organ within each nephron consists of a specialized segment of the afferent arteriole, containing juxtaglomerular cells within its wall, and the end of the thick, ascending cortical segment of the loop of Henle, the macula densa (Figure 29-7). Juxtaglomerular cells contain the enzyme renin and are innervated by the sympathetic nervous system. Release of renin depends on β1-adrenergic sympathetic stimulation, changes in afferent arteriolar wall pressure (see Chapter 49), and changes in chloride flow past the macula densa. Renin released into the bloodstream catalyzes the conversion of angiotensinogen, a protein synthesized by the liver, to angiotensin I. This inert decapeptide is then rapidly converted, primarily in the lungs, by angiotensin-converting enzyme (ACE) to form the octapeptide angiotensin II. Angiotensin II plays a major role in blood pressure regulation (see Chapter 15) and aldosterone secretion (see Chapter 49). Proximal renal tubular cells have converting enzyme as well as angiotensin II receptors. Moreover, intrarenal formation of angiotensin II enhances sodium reabsorption in proximal tubules. Some extrarenal production of renin and angiotensin II also takes place in the vascular endothelium, the adrenal glands, and the brain.
The Renal Circulation
Renal function is intimately related to renal blood flow (RBF). In fact, the kidneys are the only organs for which oxygen consumption is determined by blood flow; the reverse is true in other organs.  The combined blood flow through both kidneys normally accounts for 20-25% of total cardiac output. Approximately 80% of RBF normally goes to cortical nephrons, and only 10-15% goes to juxtamedullary nephrons. The renal cortex extracts relatively little oxygen, having an oxygen tension of about 50 mm Hg, because of the relatively high blood flow with a mostly filtration function. In contrast, the renal medulla maintains high metabolic activity because of solute reabsorption and requires low blood flow to maintain high osmotic gradients. The medulla has an oxygen tension of only about 15 mm Hg and is relatively vulnerable to ischemia.
The combined blood flow through both kidneys normally accounts for 20-25% of total cardiac output. Approximately 80% of RBF normally goes to cortical nephrons, and only 10-15% goes to juxtamedullary nephrons. The renal cortex extracts relatively little oxygen, having an oxygen tension of about 50 mm Hg, because of the relatively high blood flow with a mostly filtration function. In contrast, the renal medulla maintains high metabolic activity because of solute reabsorption and requires low blood flow to maintain high osmotic gradients. The medulla has an oxygen tension of only about 15 mm Hg and is relatively vulnerable to ischemia.
Redistribution of RBF away from cortical nephrons with short loops of Henle to larger juxtamedullary nephrons with long loops occurs under certain conditions. Sympathetic stimulation, increased levels of catecholamines and angiotensin II, and heart failure can cause redistribution of RBF to the medulla and is associated with sodium retention.