DEFINITION
Neuraxial anesthesia has predictable significant effects on respiration. These changes include altered pulmonary function, chest wall mechanics, gas exchange, and ventilatory control. There have been comparatively few studies on the respiratory effects of neuraxial anesthesia. Indeed, some often-quoted reviews on “anesthesia” and respiration fail to even mention regional anesthesia.1,2 Most of the respiratory changes associated with neuraxial anesthesia can be directly attributed to pharmacologic motor nerve blockade of respiratory musculature. In fact, neuraxial anesthesia provides a somewhat controllable experimental model for the reversible interruption of groups of respiratory muscles.
Although respiratory arrest or failure secondary to interscalene block has an extremely low incidence, the diaphragmatic paresis associated with this regional anesthetic technique results in very significant and predictable changes in respiration. Forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1) are typically reduced by 20% to 40% within 15 minutes of interscalene block injection. The diminutions in lung volumes last 3 to 5 hours with mepivacaine interscalene block and more than 9 hours with bupivacaine.3 Respiratory failure or arrest may also occur if the patient has preexisting respiratory disease,4 but more typical etiologies of acute respiratory failure associated with peripheral nerve blocks are pneumothorax5 or unintentional subarachnoid or epidural injection6–9 (Chapter 18).
 SCOPE
SCOPE
Respiratory failure is an extremely rare complication of spinal or epidural anesthesia. Acute respiratory failure during neuraxial anesthesia is most often caused by total spinal anesthesia or massive epidural anesthesia or is secondary to the effects of opiates administered into the subarachnoid or epidural space. Respiratory failure in each case is usually secondary to cephalad distribution of the anesthetic agents. The predominant etiology is alteration of respiratory control secondary to direct effect on the medulla. The incidence of respiratory depression following epidural or subarachnoid opiate administration has been published and ranges between 0.07% and 0.90%.10–16 The usual respiratory effects of neuraxial anesthesia are of negligible clinical concern in most patients (Chapters 19 and 20).
Since Urmey et al.17 first reported that hemidiaphragmatic paresis with resultant respiratory impairment occurs with an incidence of 100% following interscalene block, numerous subsequent investigators have confirmed the consistency of this side effect.18–21 Supraclavicular blocks, performed at more caudad levels than the interscalene approach, are associated with lower incidences of diaphragmatic paresis, although incidences up to 80% have been reported.22 Despite a lower incidence of hemidiaphragmatic paresis,23,24 significant alteration in respiration from supraclavicular block is variable. By contrast, most infraclavicular approaches do not have any significant effects on respiration.25,26 However, a recent study found that the vertical infraclavicular block (VIB) approach was associated with a 26% incidence of hemidiaphragmatic paralysis.27 In an original trial on the intersternocleidomastoid technique, Pham-Dang et al.28 found an incidence of reversible phrenic nerve paresis of 60%.
Pneumothorax has been associated with brachial plexus block performed by either supraclavicular or infraclavicular approaches. Other regional anesthetic techniques have been associated with pneumothorax. These include intercostal block (0.07%–19% incidence),29–32 paravertebral block (0.5% incidence),33 and intrapleural block (2% incidence).34
The extremely rare complication of permanent or persistent phrenic nerve paralysis has been reported following interscalene brachial plexus block.35–37 This presumably occurs as a result of direct trauma to the phrenic nerve by way of needle contact or intraneural injection (Box 16-1). This should be considered when performing techniques such as the intersternocleidomastoid technique,28 which involves a more anterior insertion and may traverse the course of the phrenic nerve. A recent ultrasonographic analysis of the anatomic relationship of the phrenic nerve determined that the phrenic nerve was only 2 mm away from the C5 nerve root at the level of the cricoid cartilage (Fig. 16-1). As the phrenic nerve coursed more caudally in the neck, for every centimeter increase in caudal distance, the phrenic nerve added 3 mm separation (anteriorly) from the brachial plexus. Therefore, the phrenic nerve is more likely to be avoided by techniques performed below the conventional interscalene block as described by Winnie.38
BOX 16-1 Respiratory Complications of Regional Anesthesia
Neuraxial regional anesthesia:
 Total spinal anesthesia
Total spinal anesthesia
 Massive epidural anesthesia
Massive epidural anesthesia
 Cephalad spread of opioids
Cephalad spread of opioids
Brachial plexus regional anesthesia:
 Unintended neuraxial block
Unintended neuraxial block
 Hemidiaphragmatic paresis
Hemidiaphragmatic paresis
 Permanent phrenic nerve block
Permanent phrenic nerve block
Pneumothorax
 Above-the-clavicle blocks
Above-the-clavicle blocks
 Suprascapular nerve block
Suprascapular nerve block
 Paravertebral blocks
Paravertebral blocks
 Intercostal nerve block
Intercostal nerve block
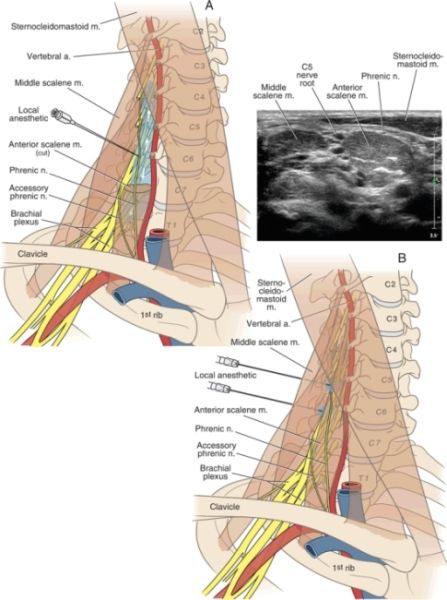
FIGURE 16-1. Panel A: When a traditional paresthesia-seeking or peripheral nerve stimulation technique is used for interscalene block, the needle is placed at the C6 level. The relatively large volumes of local anesthetic used during these techniques can spread to the cervical nerve roots, causing phrenic nerve anesthesia and hemidiaphragmatic paresis. The phrenic nerve’s overlying position on the anterior scalene muscle also exposes it to possible local anesthetic actions or needle trauma. Panel B: When ultrasound guidance is used, local anesthetic is injected at the C5 level, where the phrenic nerve is within 2 mm of the C5 nerve root (sonogram inset). This is the purported mechanism for phrenic nerve anesthesia when using ultrasound guidance, despite the ability to use a relatively reduced volume of local anesthetic.
 PATHOPHYSIOLOGY
PATHOPHYSIOLOGY
Chest Wall Muscle Paralysis
Respiratory physiologists define the “chest wall” as the rib cage, abdomen, and diaphragm. If we understand the implications of paralyzing the abdominal muscles, rib cage muscles, and diaphragm, we should be able to predict and explain the changes that occur during neuraxial anesthesia. Studies of this nature have been performed on animals following selective sectioning of nerves supplying respiratory muscles39–41 and on humans (either volunteers who relax groups of muscles42,43 or on quadriplegic patients44–46 with pathological denervation of respiratory muscles).
With more cephalad levels of epidural or spinal anesthesia, the chest wall muscles (rib cage and abdominal) are blocked, which in extreme cases may leave the diaphragm to work alone. This approximates the situation of the quadriplegic patient. Under routine neuraxial anesthesia, the main muscle of respiration, the diaphragm, remains unaffected and therefore pulmonary function is changed little. This is in contrast to other regional anesthetic techniques (e.g., interscalene block or intrapleural block), which affect the diaphragm by phrenic nerve paralysis and may therefore have more profound effects on pulmonary function and chest wall mechanics.17,19,47 Nevertheless, rib cage muscular contraction and diaphragmatic contraction coordinate during normal breathing to move the rib cage in a homogenous manner.46,48 If this coordination is interrupted by neuraxial block, chest wall deformation may occur and the characteristics of normal breathing will change.
Neuraxial Regional Anesthesia
Most clinical studies on normal patients receiving spinal or epidural anesthesia have found minimal effects on routine pulmonary function tests (PFTs).49 This was confirmed in a study of 30 patients who received epidural anesthesia with 25 to 30 mL 2% lidocaine.18 Epidural anesthesia to sensory levels averaging T5 to T6 caused mean diminution in FVC of just 176 mL (p < 0.05) and diminished peak expiratory flow rate of only 0.34 L/s (p < 0.05). Thus, although the effects of neuraxial anesthesia on the respiratory musculature and chest wall are indeed significant, routine PFTs are relatively insensitive in measuring the changes in respiratory system mechanics. This is because routine PFTs are dependent on lung mechanics and the chest wall muscle activity. In fact, FEV1 is a more clinically useful measurement in the diagnosis of lung pathology based on its dependence on the lung. FEV1 is reproducible despite variations in expiratory effort. Conversely, FEV1 or FVC is relatively insensitive for assessing alterations in respiratory muscle function and chest wall mechanics caused by neuraxial anesthesia. For instance, Sundberg et al.50 found that high thoracic epidural anesthesia at levels of T1 to T5 had little effect on FVC, decreasing it by only 300 mL. Takasaki and Takahashi51 found mean reductions in FVC after thoracic epidural anesthesia of 11%. Groeben et al.52 similarly found that thoracic epidural anesthesia in patients with significant chronic obstructive pulmonary disease (COPD) resulted in only an 8% decrease in FVC and FEV1. It must be kept in mind that, although abdominal or thoracic epidural analgesia may have a minor negative impact on respiratory function, the superior analgesia has resulted in improvement in FVC following general surgery or abdominal vascular surgery.53
Warner et al.54 studied the effects of high epidural anesthesia (approximately a T1 sensory level) on the function of the human chest wall. They found that high epidural anesthesia abolished parasternal intercostal muscle activity while preserving scalene and diaphragmatic activity. High epidural levels decreased rib cage expansion, but paradoxical (inward) motion of the rib cage occurred in only one of six study subjects. High epidural anesthesia resulted in an increase in functional residual capacity (FRC), with a significant caudad displacement of the end expiratory position of the diaphragm. However, paradoxical respiration is markedly affected by the use of intravenous sedation in conjunction with neuraxial anesthesia. Significant decreases in percentage expansion of the rib cage and in pO2 during propofol sedation during spinal anesthesia have been observed and attributed, in part, to upper airway obstruction.55
Despite sympathetic blockade, thoracic epidural anesthesia does not increase airway obstruction and only causes a small decrease in FEV1.52,56 Similarly, no significant decreases in spirometric tests were found during epidural anesthesia in parturients undergoing cesarean delivery. By contrast, Capdevila et al.57 found that cervical epidural anesthesia had significant effects on breathing pattern, diaphragmatic function, and respiratory drive in healthy patients. Cervical epidural anesthesia was associated with decreased diaphragmatic excursion, diminution in maximal inspiratory pressure up to 40.5%, and reduction in FVC up to 26.3%. Similarly, Takasaki and Takahashi51 found that cervical epidural anesthesia reduced FVC by a mean value of 28% and Michalek et al.58 found approximately a 20% diminution in FEV1 and FVC associated with cervical epidural sensory blockade from C2–C5. In a study of 324 patients undergoing carotid endarterectomy with cervical epidural anesthesia, 3 required intubation secondary to respiratory insufficiency.59
Respiratory System Mechanics
To understand the alterations in respiration that result from pharmacologic motor nerve blockade during epidural anesthesia, one must first appreciate the basic mechanics underlying normal chest wall motion and normal (nonparalyzed) respiratory muscle actions. These factors have been clarified through the work of Mead, De Troyer, and other respiratory physiologists. Goldman and Mead43 first described the pressure-volume characteristics of the chest wall by using magnetometers to make indirect measurements of volume changes of the two major compartments of the chest wall: (i) the rib cage (RC) and (ii) the abdomen-diaphragm (AB-Di). They measured the pressure driving these compartments by dividing the chest wall into three parts: (i) the RC, (ii) the AB, and (iii) the diaphragm. The following equations algebraically describe the transmural pressures across each part.
[16.1] 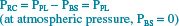
[16.2] 
[16.3] 
Here, PRC = the transmural pressure driving the rib cage, PPL = pleural pressure, PBS = body surface pressure, PABW = pressure across the abdominal wall, PAB = intraabdominal pressure, and PDI = transdiaphragmatic pressure.
Although the active chest wall has mainly two degrees of freedom of motion,41 the relaxed chest wall (all muscles voluntarily relaxed) has only one set of corresponding pressures and volumes. Thus, a “relaxation pressure-volume curve” could be generated for the voluntarily relaxed chest wall. Goldman and Mead noticed that quiet breathing followed this relaxed characteristic curve. To “stress the system” and make transmural driving pressure change, a large abdominal cuff was inflated to passively squeeze the abdomen of volunteers who relaxed their respiratory muscles. Each cm H2O change in transdiaphragmatic pressure (PDI) resulted in a decrease in pleural pressure (PPL) of an equal magnitude. In addition, the increasing abdominal pressure expanded the rib cage. This can be understood algebraically by the examination of the following equations.
[16.4] 
Substituting Equation 16.3 yields:
[16.5] 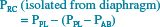
[16.6] 
Thus, the pressure driving the rib cage in the absence of the diaphragm is intraabdominal pressure. Because diaphragmatic contraction increases the intraabdominal pressure (PAB), Goldman and Mead43 erroneously concluded that the diaphragm was the only active muscle during tidal breathing.
It later became evident from studies in quadriplegics46 that true isolated diaphragmatic action expands only the lower rib cage (LRC). The upper rib cage (URC) is pulled inward by decreasing pleural pressure, which deforms the rib cage overall. This is similar to what had been described during high levels of spinal anesthesia by Eisele et al.60 in 1968. They observed that during continuous spinal anesthesia to attain a T1 motor level, “the lower half of the chest moved out with inspiration while the upper half passively retracted.”
Mead proposed a mechanism to explain these new findings by considering the diaphragm’s geometry. The zone of apposition (Zap) allows for the direct application of PAB to the inner rib cage wall such that increased PAB by diaphragmatic contraction is directly applied to the inner RC wall through the Zap.61 Urmey et al.62 confirmed this theory in animal studies. Two components of diaphragmatic action on the LRC have thus been identified (Fig. 16-2):
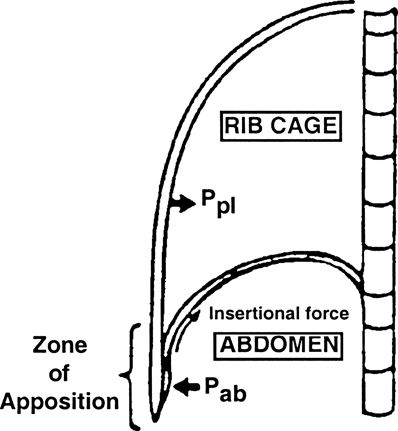
FIGURE 16-2. Lateral schematic illustration of the chest wall. (Ppl, pleural pressure; Pab, abdominal pressure.) (Adapted from Green N. Physiology of Spinal Anesthesia, Pulmonary Ventilation and Hemodynamics. Baltimore, MD: Williams & Wilkins, 1981.)
 An insertional component, whereby the directional vector of diaphragm insertions and the mechanics of rib/vertebral articulations result in RC elevation and expansion
An insertional component, whereby the directional vector of diaphragm insertions and the mechanics of rib/vertebral articulations result in RC elevation and expansion
 An appositional component, whereby increases in PAB are transmitted through the Zap to expand the rib cage
An appositional component, whereby increases in PAB are transmitted through the Zap to expand the rib cage
Both of these effects are only on the LRC. Decreasing pleural pressure passively sucks the URC inward in the absence of intercostal muscle function. This explains the characteristic deformation of the rib cage during epidural anesthesia, spinal anesthesia,63,64 and quadriplegia (Fig. 16-3).
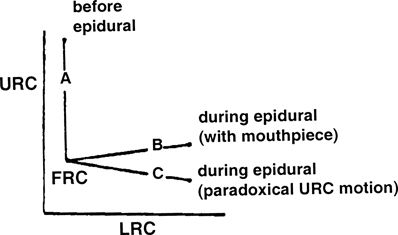
FIGURE 16-3. Upper rib cage (URC) and lower rib cage (LRC) expansion before and during epidural anesthesia. (FRC, functional residual capacity.) (Reproduced from Urmey W, Lambert D, Concepcion M. Routine spinal or epidural anesthesia causes rib cage distortion during spontaneous inspiration (abstract). Anesthesiology 1987;67:A538, with permission.)
Diminished Cough Strength
Spinal or epidural anesthesia causes a level-dependent compromise in the ability to effectively cough. Unlike routine PFTs, the ability to cough effectively is dramatically and significantly affected by neuraxial anesthesia. In one study, 0.75% bupivacaine lumbar epidural anesthesia to levels of T3 to T4 caused an approximately 50% reduction in peak inspiratory pressure during cough (Fig. 16-4).65 Egbert et al.66 found a 53% reduction in intraabdominal pressure during cough in patients who had received spinal anesthesia. With ascending levels of neuraxial anesthesia, thoracoabdominal muscles are increasingly paralyzed.
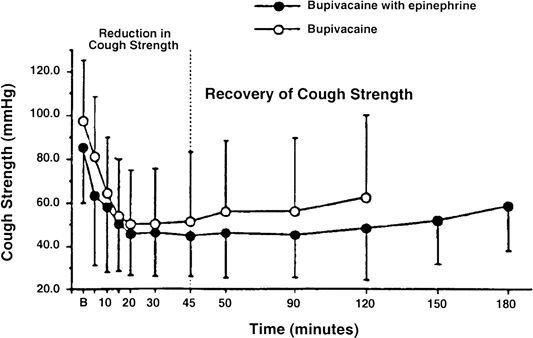
FIGURE 16-4. Reduction of cough strength as a function of time after epidural local anesthetic injection. (Reproduced with permission of Urmey WF. Case studies of regional anesthesia. In: Complications of Regional Anesthesia. New York, NY: Churchill-Livingstone, 1999.)



