V: MANAGEMENT OF POSTPARTUM COMPLICATIONS COMMONLY SEEN IN OBSTETRIC TRIAGE |
|
Postpartum Preeclampsia Complications | 28 |
Hypertensive disorders are one of the most common causes of presentation for emergency care in the postpartum period, ranking second to infections (Clapp, Little, Zheng, & Robinson, 2016). Although many women experience hypertensive disorders during pregnancy, either due to chronic disease or to the spectrum of gestational hypertension and preeclampsia, de novo development of hypertension is seen in 42% to 77% of women with postpartum hypertension (Al-Safi et al., 2011; Goel, 2015). Eclampsia is one of the most concerning complications of hypertensive disorders of pregnancy and contributes significantly to maternal mortality. In Canada, a case fatality rate of 3.4 per 1,000 deliveries was noted from 2003 to 2009, making the risk of death from eclampsia 26.8 times greater than those who do not experience eclampsia (Liu et al., 2011). In addition, eclampsia is associated with increased risk of morbidity including the need for assisted ventilation, adult respiratory distress syndrome, acute renal failure, and cardiac arrest (Liu et al., 2011).
Significant research and resources have been devoted to the problem, leading to a marked reduction of eclampsia in the antenatal period. However, women with hypertensive disorders in the postpartum period have been excluded from this research (Sibai, 2011). As women will frequently manifest problems due to postpartum hypertension following discharge from the hospital, they are often initially managed in an obstetric triage or emergency room setting. Of women who presented with seizure in the postpartum period, more than 90% presented within 7 days of the original hospital discharge (Al-Safi et al., 2011). While women with mild preeclampsia are 25 times more likely to experience hypertension during the postpartum period, hypertensive disorders, including eclampsia, do initially present in the postpartum period (Cruz, Gao, & Hibbard, 2011).
It is difficult to define the incidence of hypertensive disorders of pregnancy presenting in the postpartum period. Overall, the incidence of new-onset hypertensive disorders in the postpartum period ranges from 0.3% to 27.5% (Sibai, 2011). In a 10-year review of 3,899 cases of preeclampsia, 5.7% of cases were initially diagnosed in the postpartum period, of which 66% occurred after the original discharge date (Matthys, Coppage, Lambers, Barton, & Sibai, 2004). Chames, Livingston, Ivester, Barton, and Sibai (2002) noted that 79.3% of women with eclampsia in the postpartum period presented late (>48 hours after delivery), and of these, only 22% had a history of preeclampsia in the 334index pregnancy. Al-Safi found 63% of women with postpartum preeclampsia and 77.3% of those with eclampsia had no antecedent history of hypertensive disorder of pregnancy (Al-Safi et al., 2011). Similarly, a review of 988 deliveries found 184 women presented for evaluation of postpartum hypertension, and of these, 77 cases were new onset in the postpartum period (Goel et al., 2015).
Traditionally, the postpartum period has been considered to extend into the fourth postpartum week (Yancey, Withers, Bakes, & Abbot, 2011). After discharge, many women are not seen in follow-up until 6 weeks and may not present earlier unless they experience symptoms. Women with the antenatal diagnosis of pregnancy-induced hypertensive disorders do not normalize blood pressure immediately following delivery. In one study of 62 patients, 81% had normalized by 3 months postpartum and required a mean of 5.4 weeks to reach normal blood pressures (Podymow & August, 2010). However, there may be a brief 48-hour window following delivery of normal blood pressures followed by an increase in blood pressure between postpartum days 3 to 6, likely due to physiologic volume expansion and fluid mobilization (American Congress of Obstetricians and Gynecologists [ACOG], 2013; Ghuman, Rheiner, Tendler, & White, 2009; Podymow & August, 2010). As most women are discharged home prior to the fifth postpartum day in which blood pressure has been shown to reach peak values, the need for possible postpartum blood pressure treatment may not be identified (Podymow & August, 2010). Given this, blood pressure evaluation at 72 hours postpartum, and then again 7 to 10 days postpartum, is recommended for women with known hypertensive disorders of pregnancy (ACOG, 2013).
Rates of eclampsia have been declining. In Canada, the rate of eclampsia was noted to fall from 12.4 per 10,000 deliveries in 2003 to 5.9 in 2009 (Liu et al., 2011). While there has been reduction in the incidence of antenatal eclampsia due to improved prenatal care, screening, and prophylactic treatment with magnesium sulfate, in the past 60 years there has been no decrease in the incidence of postpartum eclampsia, with 14% to 26% of eclamptic seizures noted to occur greater than 48 hours after delivery (Chames et al., 2002; Liu et al., 2011; Yancey et al., 2011).
It is critical to quickly evaluate and treat those who present with elevated blood pressure, especially if associated with prodromal symptoms such as headache. Headache was the most common presenting symptom in women with delayed postpartum preeclampsia in Al-Safi’s series, present in 69.1% (Al-Safi et al., 2011). In Chames’s series, 91% of women with postpartum eclampsia were noted to have prodromal symptoms. Only seven sought care prior to the onset of seizure activity, but six of these women were deemed to have had a preventable seizure due to failure to consider a diagnosis of preeclampsia in the postpartum setting (Chames et al., 2002). Since the differential diagnosis of postpartum hypertension is broad, a careful history and physical examination must elucidate the etiology and appropriate management.
PRESENTING SYMPTOMATOLOGY
Although it is unclear how many women with elevated blood pressure are not evaluated postpartum due to lack of symptoms, it is clear that those with postpartum eclampsia will likely experience symptoms. Women with 91% to 100% of postpartum eclampsia are noted to have prodromal symptoms (Al-Safi et al., 2011; Chames et al., 2002). In multiple studies, headache has been shown to be the most common symptom (Al-Safi et al., 2011; Chames et al., 2002; Matthys et al., 2004; Yancey et al., 2011). Other common symptoms are displayed in Table 28.1.
TABLE 28.1 Common Symptoms Associated With Postpartum Preeclampsia
SYMPTOM | PERCENTAGE OF POSTPARTUM WOMEN WITH PREECLAMPSIA (%) |
Headache | 62–82 |
Visual changes | 19–31 |
Shortness of breath/chest pain | 13–30 |
Nausea | 12.5–18 |
Vomiting | 11.2–14 |
Abdominal pain | 7–14 |
Edema | 9–10.5 |
Neurologic deficits | 5.3 |
Sources: Al-Safi et al. (2011); Matthys et al. (2004); and Yancey et al. (2011).
It is essential to note that while headache is the most common presenting symptom, and headache with associated elevated blood pressure prompts evaluation for preeclampsia, headache itself is nonspecific and is associated with a broad differential. Hypertensive disorders of pregnancy represent 24% of postpartum headaches, second most common behind tension-type headaches (Stella, Jodicke, How, Harkness, & Sibai, 2007).
HISTORY AND DATA COLLECTION
Given the broad differential associated with postpartum hypertension, a thorough history is critical. In addition to talking with the patient, prenatal and hospital admission records are obtained and reviewed to obtain the following: determination of the interval between delivery and clinical presentation, a thorough medical and pregnancy history, determination of associated symptoms, family history with particular attention to cerebrovascular accidents, and confirmation of medication usage.
The time course of postpartum hypertensive disorders has classically been defined to extend into the fourth postpartum week, but postpartum hypertensive disorders primarily present within the first week (Yancey et al., 2011). In Goel’s study, 62% of women presented between 48 and 72 hours postpartum, 27.2% presented on day 4, 5.4% on day 5, and the remaining after the first week (Goel et al., 2015). It is the most common reason for readmission on day 3 postpartum (Clapp et al., 2016). After 6 weeks postpartum, an alternative diagnosis, especially essential hypertension, is considered. While it is critical to know about the antenatal history, many cases of hypertension postpartum, especially eclampsia, have not been previously diagnosed. In a series of 152 cases readmitted for delayed postpartum preeclampsia or eclampsia, 96 cases (63.2%) had no antecedent diagnosis, and in a separate study of 184 women seen for hypertensive disorders in the postpartum period, 77 (42%) were de novo cases (Al-Safi et al., 2011; Goel et al., 2015).
Some studies have noted that African American women are at higher risk for the development of eclampsia, but specific risk factors have not been well established for the development of postpartum preeclampsia or eclampsia, as they have been for the antepartum period (Al-Safi et al., 2011; Matthys et al., 2004). One series evaluated 1,964 women and found independent risk 336factors for development of postpartum hypertension which included: assisted reproductive technology, obesity, chronic nephritis, hypothyroidism, high normal blood pressure before or at delivery, and cesarean section (Takaoka et al., 2016). Chronic hypertension itself does not appear to be a risk factor for postpartum eclampsia. Of 543 women with chronic hypertension with superimposed preeclampsia, only 5.2% were readmitted postpartum and none developed eclampsia (Al-Safi et al., 2011). In the same study that found women with mild preeclampsia had 25 times the risk of having elevated pressures postpartum, women with chronic hypertension were only seven times more likely than women without hypertensive disorders to have elevated blood pressure postpartum (Cruz et al., 2011). However, while women with chronic hypertension were six times more likely to have a seizure as compared with normal controls, those with preeclampsia had only four times the risk (Cruz et al., 2011). Ultimately, knowledge of any underlying history may help to distinguish worsening essential hypertension from developing preeclampsia.
Other associated symptoms may be clues that an alternative diagnosis or diagnoses must be considered. Hyperthyroidism, either Graves’ disease or the hyperthyroid phase of postpartum thyroiditis, may present with palpitations and heat intolerance. Shortness of breath and chest pain are associated with peripartum cardiomyopathy, of which 23% to 46% of cases have associated hypertension (Sibai, 2011). Symptoms of other cerebrovascular complications often overlap with those of preeclampsia and include refractory or thunderclap headache (a sudden onset pain often described as the worst headache a woman has ever experienced), visual disturbances, or neurologic deficits (Stella et al., 2007). Pheochromocytoma, although a rare entity, is associated with high morbidity and symptoms such as palpitations, excessive sweating, chest pain, and dizziness (Sibai, 2011).
Medication usage is an essential part of the history. The use of nonsteroidal anti-inflammatory medications like ibuprofen is common practice. These medications have been shown to lead to hypertension by reducing compensatory renal prostaglandin synthesis in hypertensive patients while concomitantly increasing renal synthesis of vasoconstricting agents (Makris, Thronton, & Hennessy, 2004). They may also inhibit salt and water loss postpartum as well as decrease the effectiveness of many antihypertensive drugs including beta blockers, angiotensin-converting enzyme inhibitors, and thiazide diuretics (Ghuman et al., 2009). Anticongestants such as ephedrine and phenylpropanolamine are other commonly used medications associated with exacerbation of hypertension. Ergot alkaloids like methylergonovine, administered for uterine atony in the postpartum period, cause vasoconstriction by acting on alpha adrenergic receptors and therefore can contribute to the hypertensive issues (Sibai, 2011).
PHYSICAL EXAMINATION
Potential life-threatening complications of postpartum hypertension include cerebral infarction or hemorrhage, congestive heart failure, pulmonary edema, or renal failure (Sibai, 2011). Signs of these processes may be detected while performing a thorough physical examination. Vital signs alone may provide clues as to the etiology of the hypertension. Acute onset, severe hypertension (defined as systolic pressure greater than or equal to 160 mmHg or diastolic pressure greater than or equal to 110 mmHg) that persists greater than 15 minutes is a hypertensive emergency. The degree of systolic hypertension appears to be the most significant predictor of cerebral injury and infarction. In a series of 28 patients with preeclampsia and stroke, all but one had severely elevated 337systolic pressures just prior to experiencing hemorrhagic stroke, while only 13% had severely elevated diastolic pressures (ACOG, 2015). Widened pulse pressure and tachycardia may be seen with hyperthyroidism, postural hypotension with pheochromocytoma, and decreased oxygen saturation with congestive heart failure or pulmonary edema (Sibai, 2011).
A thorough neurologic examination including evaluation of cranial nerves, visual fields, motor strength, sensation, cerebellar function, reflexes, and presence of clonus must be performed. Brisk reflexes, especially if associated with clonus, are classically associated with a preeclamptic state. Forty-seven percent of women presenting with postpartum hypertension or eclampsia were noted to have hyperreflexia (Yancey et al., 2011). It is critical to urgently evaluate any neurologic deficits as they are indicative of possible serious intracranial abnormalities.
Cardiovascular and respiratory examination may reveal possible fluid overload, which is associated with pulmonary edema; this is not an uncommon complication of both preeclampsia and peripartum cardiomyopathy. Abdominal examination may reveal right-upper-quadrant tenderness. In Yancey’s study of women presenting with postpartum preeclampsia or eclampsia, 18% were noted to have this finding (Yancey et al., 2011). Although it is a very common finding seen in as high as 84% of women presenting with postpartum hypertension, edema (generalized or peripheral) is a nonspecific finding (Yancey et al., 2011). The presence may be noted but may not help elucidate etiology.
LABORATORY AND IMAGING STUDIES
A complete laboratory panel upon presentation consists of a complete blood count, liver enzymes, serum creatinine, and electrolytes. As proteinuria is only seen in 29% to 79% of postpartum eclampsia, and as the degree of proteinuria does not determine the severity of preeclampsia, assessment of the presence of proteinuria is less critical. While the presence strongly suggests preeclampsia as an etiology, its absence does not rule out preeclampsia and possible impending eclampsia (ACOG, 2013; Yancey et al., 2011). In addition, as normal postpartum lochia may influence the presence of proteinuria, it is necessary to obtain the sample by catheterization.
Hemolysis, elevated liver enzymes, and low platelet (HELLP) syndrome, another serious complication of preeclampsia, has also been noted to initially present in 30% of women who develop this syndrome from postpartum days 1 through 7 (Sibai, 2011). In Yancey’s study of 22 women with postpartum hypertension or eclampsia, 41% had elevated liver enzymes, 24% had anemia, but none were noted to have thrombocytopenia (Yancey et al., 2011).
As with physical examination findings, other laboratory findings may help to identify alternative etiologies of hypertension. For example, serum potassium levels less than 3 mEq/L with associated metabolic acidosis are indicative of hyperaldosteronism. Confirmation of adrenal tumor may then be made with either computerized tomography (CT) or magnetic resonance imaging (MRI) of the abdomen (Sibai, 2011). Low serum creatinine is suggestive of volume overload (Sibai, 2011).
Presenting symptoms and physical examination findings concerning for pheochromocytoma include: paroxysmal hypertension associated with headache, profuse sweating, palpitation, tachycardia, pallor, and possible fever. Measurement of 24-hour urine epinephrine, norepinephrine, and metabolites (metanephrine and normetanephrine) can lead to the diagnosis, which is then confirmed with either CT or MRI of the abdomen (Sibai, 2011). Symptoms and physical examination findings that point to pulmonary edema necessitate further evaluation with chest x-ray and possible echocardiography (Sibai, 2011).
338In the antepartum evaluation of hypertensive disorders of pregnancy, neurodiagnostic imaging is rarely indicated. However, in the postpartum period, the differential diagnosis is wider, especially as distinguishing laboratory findings, such as proteinuria, are not always present. In addition, treatment modalities may be dictated by diagnosis made by imaging. Different studies have examined the utility of neurodiagnostic imaging. In Yancey’s series of 22 women with postpartum hypertension or eclampsia, 40.9% underwent head CT, and of these, three had significant findings including diffuse edema, cerebellar hypodensities, and small white matter hypodensities (Yancey et al., 2011). In the evaluation of postpartum headache, Stella found normal imaging in only 32% by employing indications including focal neurologic deficits, new-onset seizures, recurrent seizures despite prophylactic magnesium sulfate, persistent visual changes, or persistent refractory headache (Stella et al., 2007). These abnormal findings included pituitary hemorrhage, posterior reversible encephalopathy syndrome (PRES), cerebral venous thrombosis, inflammatory changes, thalamic lesions, and subarachnoid hemorrhage (Stella et al., 2007). Ultimately, neurodiagnostic imaging studies aimed at evaluating the cerebral vasculature—MRI and arterial and venous angiography—will need to be obtained if these findings are present.
PRES is commonly associated with eclampsia and is clinically characterized by acute onset headache, altered mental status, cortical blindness, and seizures with parietooccipital involvement (Cozzolino et al., 2015; Stella et al., 2007). Primarily occurring in the setting of preeclampsia, one theory of pathophysiology is that severe hypertension exceeds the limits of autoregulation, resulting in brain edema (Lamy, Oppenheim, & Mal, 2014). In addition, in preeclampsia, endothelial cells are damaged and this pressure imbalance leads to vasogenic edema (Bushnell & Chireau, 2011). MRI is the gold standard for diagnosis (Cozzolino et al., 2015). Classic findings of edema in the parietooccipital white matter are seen in Figure 28.1, which shows extensive areas of subcortical increased T2 signal with enhanced diffusivity involving posterior parietal and occipital white matter.
Reversible cerebral vasoconstrictive syndrome is a similar condition, classically presenting with a thunderclap headache, which is more severe and 339sudden than that associated with PRES. Although the pathophysiology is unclear, it is likely due to a disturbance in the control of cerebral vascular tone (Bushnell & Chireau, 2011). Other possible intracranial etiologies and associated radiographic findings are displayed in Table 28.2.
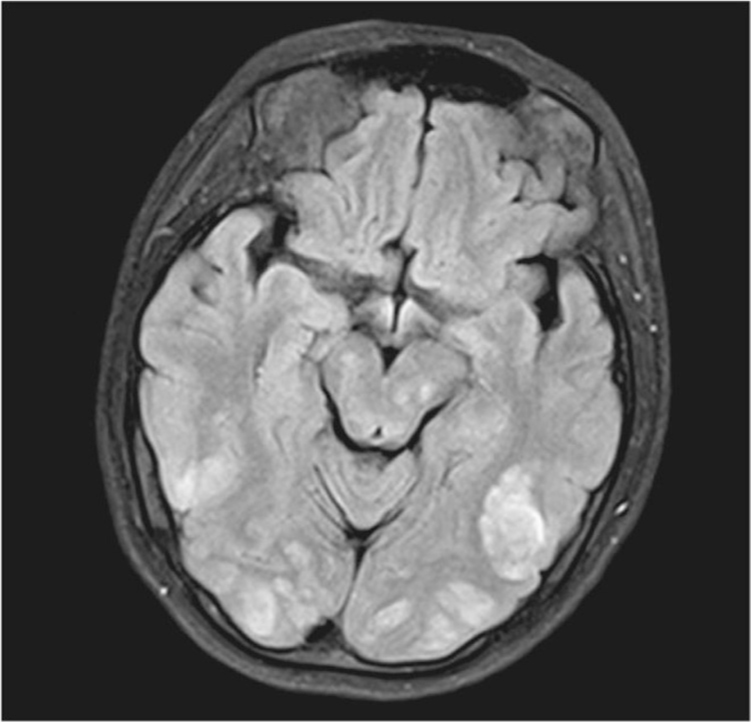
Figure 28.1 MRI findings in PRES
Source: Courtesy of Radiology Department, Women & Infants Hospital, Providence, RI.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








