Male gender
 Tobacco use
Tobacco use
 Increasing age
Increasing age
 Preexisting diabetes mellitus
Preexisting diabetes mellitus
 Extremes of body habitus
Extremes of body habitus
 Preexisting neurologic disorders
Preexisting neurologic disorders
Surgical Risk Factors
 Surgical trauma or stretch
Surgical trauma or stretch
 Tourniquet ischemia
Tourniquet ischemia
 Vascular compromise
Vascular compromise
 Perioperative inflammation
Perioperative inflammation
 Hematoma
Hematoma
 Cast compression or irritation
Cast compression or irritation
 Postoperative infection or abscess formation
Postoperative infection or abscess formation
 Patient positioning
Patient positioning
Anesthetic Risk Factors
 Needle- or catheter-induced mechanical trauma
Needle- or catheter-induced mechanical trauma
 Intrafascicular injection
Intrafascicular injection
 Ischemic injury (vasoconstrictors)
Ischemic injury (vasoconstrictors)
 Perineural edema
Perineural edema
 Local anesthetic toxicity
Local anesthetic toxicity
 DEFINITION
DEFINITION
Perioperative nerve injury may be defined as the presence of pain, paresthesias, sensory or motor deficits, or other neurologic abnormalities (e.g., cranial nerve dysfunction) after surgery. The deficit(s) may be newly diagnosed (i.e., no prior history) or a progression of preexisting neurologic findings. A persistent neurologic deficit occurring after regional anesthesia refers to a sensory or motor abnormality that lasts beyond the expected duration of an administered local anesthetic. While the majority of perioperative neurologic deficits are transient and self-limited, those remaining unchanged after 12 months are considered permanent by many neurologists and neurosurgeons.
 SCOPE
SCOPE
Background and Incidence
Several investigators have examined the incidence of perioperative nerve injury with varying results. For example, during the past decade, prospective randomized studies have reported the frequency of perioperative nerve injury after regional anesthesia to range from 0.02%5 to 11%.6 Several differences in study methodology likely account for the tremendous variation in reported rates of neurologic complications (Box 14-2).7
BOX 14-2 Variables Contributing to Differences in Rates of Perioperative Nerve Injury
Sample sizes
 Small vs. large studies
Small vs. large studies
Surgical procedures and block techniques
 Multiple surgical procedures vs. single surgical intervention
Multiple surgical procedures vs. single surgical intervention
 Upper extremity block techniques vs. lower extremity block techniques vs. both
Upper extremity block techniques vs. lower extremity block techniques vs. both
Providers performing the blocks
 Experienced vs. novice
Experienced vs. novice
Patient status during block placement
 Awake vs. sedated vs. general anesthesia
Awake vs. sedated vs. general anesthesia
Exclusion criteria
 Variable (may or may not include patients with preexisting neurologic deficits)
Variable (may or may not include patients with preexisting neurologic deficits)
Definition of perioperative nerve injury
 New deficits vs. worsening of preexisting deficits vs. both
New deficits vs. worsening of preexisting deficits vs. both
 Anesthesia-related injuries vs. surgical related injuries vs. both
Anesthesia-related injuries vs. surgical related injuries vs. both
Method of postoperative evaluation
 Direct contact by anesthesiologist vs. patient self-reports vs. surgical follow-up vs. voluntary patient or provider surveys
Direct contact by anesthesiologist vs. patient self-reports vs. surgical follow-up vs. voluntary patient or provider surveys
Time of follow-up evaluation
 Variable (48 hours to 6 months)
Variable (48 hours to 6 months)
Welch et al.8 examined the frequency of perioperative nerve injury in 380,680 surgical cases at a single institution over a 10-year period of time. Overall, they identified a neurologic complication rate of 0.03%. General and epidural anesthesia were more often associated with neurologic injury than spinal anesthesia or peripheral nerve blockade (PNB). Significant associations were also found among patients undergoing neurosurgical interventions, cardiac surgery, general surgery, and orthopedic surgery. In contrast, Jacob et al.9 recently found that the type of anesthesia (general vs. neuraxial) or PNB was not associated with an increased risk of perioperative nerve injury in 12,329 patients undergoing total knee arthroplasty.
Auroy et al.10 performed one of the first large-scale prospective investigations evaluating the risk of serious complications related to regional anesthesia. In this French survey, a total of 103,730 regional anesthetics, including 71,053 neuraxial anesthetics, 21,278 peripheral nerve blocks (PNB), and 11,229 intravenous regional anesthetics, were performed over a 5-month period of time. Neurologic complications related to regional anesthetic techniques occurred in 34 (0.03%) patients. This represented 26% of all complications related to regional anesthesia. Of the 34 neurologic complications, 24 (70%) occurred during spinal anesthesia, 6 (18%) during epidural anesthesia, and 4 (12%) during PNB. All neurologic complications occurred within 48 hours of surgery and resolved within 3 months in 85% of patients. In all cases of neurologic injury after PNB, needle placement was associated with either a paresthesia during needle insertion or pain on injection. In all cases, the sensory deficit had the same topography as the associated paresthesia during block placement.
In a follow-up investigation, Auroy et al.5 further examined 158,083 regional anesthetics—including 50,223 PNB—over a 10-month period. The overall incidence of serious complications related to regional anesthesia (all techniques) was found to be approximately 4/10,000 (0.04%) (Table 14-1). Interestingly, when they specifically examined the risk associated with PNB, they found an identical incidence of 4/10,000 (0.04%). However, a significantly higher percentage of complications were noted with the posterior lumbar plexus block, when compared to other peripheral techniques (Table 14-2). Similar to their initial findings, the incidence of cardiac arrest and neurologic complications was found to be significantly higher after spinal anesthesia when compared to other types of regional anesthesia (Table 14-1). Neurologic complications attributed specifically to the regional technique occurred in 26 (0.02%) patients; recovery was complete within 6 months in 16 (62%) of the 26 patients. Of the 12 patients experiencing a neurologic complication after PNB, 7 (58%) persisted for >6 months. Of these, nine had been performed with the use of a peripheral nerve stimulator.
TABLE 14.1 Serious Complications Related to Regional Anesthesia
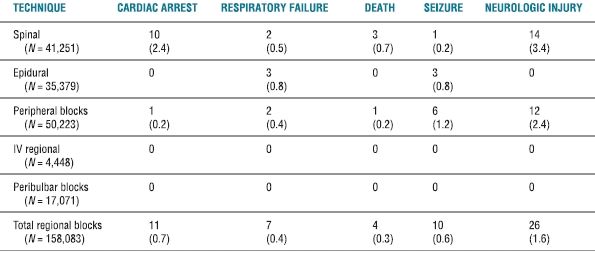
Data presented are number and the estimated (n/10,000) where applicable.
Reprinted from Auroy Y, Benhamou D, Bargues L, et al. Major complications of regional anesthesia in France: the SOS Regional Anesthesia Hotline Service. Anesthesiology 2002;97:1274–1280, with permission.
TABLE 14.2 Serious Complications Related to PNB
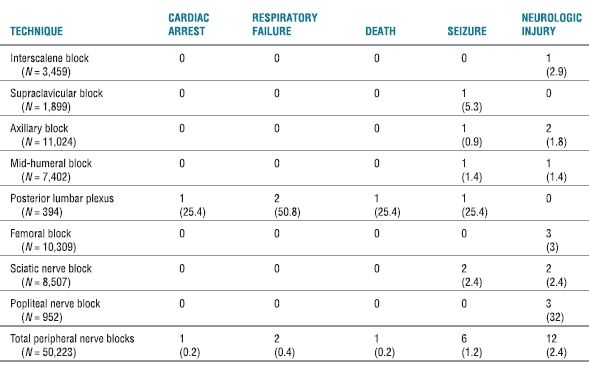
Data presented are number and the estimated (n/10,000) where applicable.
Reprinted from Auroy Y, Benhamou D, Bargues L, et al. Major complications of regional anesthesia in France: the SOS Regional Anesthesia Hotline Service. Anesthesiology 2002;97:1274–1280, with permission.
More recently, Barrington et al.11 performed a prospective audit of more than 7,000 PNB performed in nine Australian hospitals. Ultrasound imaging was used as the primary mode of neural localization in more than 63% of the procedures. Overall, they identified a neurologic injury rate of 0.5%. However, only 10% of these injuries were attributed to PNB, suggesting that the vast majority of perioperative nerve injuries have a nonanesthesia-related etiology. The nerve injury rate attributed to PNB was found to be 0.04%—a rate similar to other large-scale investigations.5,10 Horlocker et al.12 have also found that nearly 90% of perioperative nerve injures are secondary to surgical (i.e., nonanesthesia) causes. Of the patients experiencing neurologic deficits secondary to regional anesthesia, the vast majority (66%) had their deficits persist beyond 6 months, with 33% of deficits extending for >1 year.11 Jacob et al.9 have suggested that anesthesia-related neurologic deficits (i.e., deficits occurring after PNB) may be less likely to have a complete neurologic recovery when compared to nerve injuries from surgical causes.
Many investigators speculate that the risk of peripheral nerve injury may be higher in patients undergoing continuous perineural catheter placement versus single-injection techniques. The frequency of minor neurologic deficits (e.g., hypoesthesias, dysesthesias, persistent numbness) occurring within the first few days after perineural catheter placement is variable, ranging from 0%13 to 8%.14 However, severe neural lesions or long-term (i.e., 3–6 months) neurologic deficits occur at rates comparable to single-injection techniques.13–18
Based upon these and other findings, most authors conclude that needle-induced mechanical trauma and local anesthetic neurotoxicity are the primary etiologies of neurologic complications attributed to regional anesthesia. Although most of these studies found that the incidence of severe anesthesia-related complications is extremely low, they have also demonstrated that serious complications may occur in the presence of experienced anesthesiologists—and that continued vigilance in patients undergoing regional anesthesia is not only warranted, but critical to minimize perioperative nerve injuries.
Closed Claims Analysis
Cheney et al.19 examined the American Society of Anesthesiologists Closed Claims database to further delineate the role of nerve damage in malpractice claims filed against anesthesia care providers. Of the 4,183 claims reviewed, 670 (16%) were for anesthesia-related nerve injury. The most frequent sites of injury were the ulnar nerve (28%), the brachial plexus (20%), the lumbosacral nerve roots (16%), and the spinal cord (13%). Additional mononeuropathies comprised the remaining 22% of claims. Overall, regional anesthesia was more frequently associated with nerve damage claims when compared to general anesthesia. The only exception was ulnar nerve injuries, which were predominantly associated with general anesthesia (85%).
Regional anesthetic techniques (axillary, interscalene, and supraclavicular blockade) were attributed specifically to 16% of all brachial plexus injuries, in which 31% experienced a paresthesia either during needle placement or with injection of local anesthetic. In contrast, 30% of ulnar nerve injuries were attributed to regional anesthetic techniques in which a mechanism of injury was explicitly stated. Interestingly, none of these claims involved the elicitation of a paresthesia. In these cases, the onset of symptoms occurred immediately within the postoperative period in 21% of patients and was delayed from 1 to 28 days postoperatively (median 3 days) in 62% of cases.19 It has been suggested that neurologic deficits that arise within the first 24 hours of surgery most likely represent extra- or intraneural hematoma, intraneural edema, or a lesion involving a sufficient number of nerve fibers to allow immediate diagnosis.12,20 In contrast, several investigations have reported a delay in neurologic findings, in which symptoms develop several days or even weeks following surgery.10,12,19–21 The presentation of late disturbances in nerve function may suggest an alternate etiology, such as a tissue reaction, scar formation, or inflammatory process leading to the degeneration of nerve fibers.20,22 However, it is not possible from the available data to determine whether this reaction may be due to mechanical trauma, local anesthetic neurotoxicity, or a combination of both.
In a more recent review of the American Society of Anesthesiologists Closed Claims database, Lee et al.23 specifically examined serious injuries associated with regional anesthesia during the period 1980 to 1999. During this time, a total of 1,005 regional anesthesia claims were identified, of which 368 (37%) were obstetric related claims and 637 (63%) nonobstetric claims. All obstetric claims were related to complications associated with neuraxial anesthesia or analgesia. In contrast, 134 of the 637 (21%) nonobstetric claims were related specifically to complications occurring during PNB. An updated review of the Closed Claims database reported that axillary blocks were used in the majority of peripheral nerve block claims (40%), followed by interscalene blocks (23%), intravenous regional anesthesia (19%), supraclavicular blocks (8%), intercostal nerve blocks (2%), cervical plexus blocks (2%), and ankle blockade (2%).24 Nerve damage was associated with 59% of peripheral nerve block claims and was evenly divided between temporary and permanent injury. The damaging event was related to the block technique in over 50% of cases. Overall, temporary or permanent peripheral nerve injury occurred in 79 (59%) of 134 peripheral nerve claims. Upper extremity techniques were more commonly associated with peripheral nerve claims than lower extremity techniques.23,24 Additional complications occurring during PNB included death or brain damage (13%), pneumothorax (10%), emotional distress (2%), inflammatory skin reactions (2%), and miscellaneous causes (14%).23 Peripheral nerve block claims were associated with substandard care in 21% of claims.24
 PATHOPHYSIOLOGY
PATHOPHYSIOLOGY
Peripheral Nerve Anatomy
The peripheral nervous system (PNS) comprises <0.1% of all nerve tissue. The somatic PNS, when defined anatomically by the presence of Schwann cells, includes the primary nerve roots, dorsal root ganglions, mixed spinal nerves, plexuses, nerve trunks, and the autonomic nervous system. Each peripheral nerve is composed of individual myelinated nerve fibers embedded within an endoneurial connective tissue layer and grouped into discrete bundles termed fascicles. Each fascicle is surrounded by a squamous epithelial sheath called the perineurium that regulates the microenvironment, or homeostatic milieu, of the myelinated nerve fibers. Nerve fascicles are surrounded by a connective tissue environment (i.e., perineural space) and encased by the peripheral nerve’s outer epineurial membrane (Fig. 14-1).
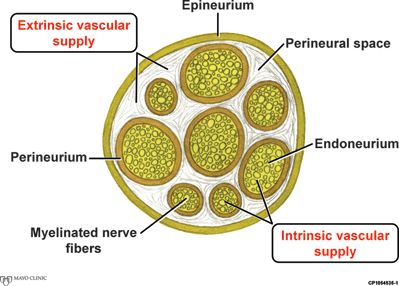
FIGURE 14-1. Cross-sectional peripheral nerve anatomy. Cross-section of a peripheral nerve with its vascular supply. (By permission of Mayo Foundation for Medical Education and Research.)
The PNS consists of both sensory and motor components. The cell bodies of sensory nerves are located within the dorsal root ganglion, while those for motor nerves are located within the anterior horn of the spinal cord. The normal function of myelinated nerve fibers depends upon the integrity of both the axon and its myelin sheath. Nerve action potentials “jump” from one node of Ranvier to the next—propagating neural signal transmission. This rapid saltatory conduction depends on the insulating properties of the myelin sheath for proper and efficient functioning.
The functional integrity of a peripheral nerve is highly dependent upon its microcirculation,25 which consists of an intrinsic supply of exchange vessels within the endoneurium and an extrinsic supply of larger, non-nutritive vessels located within the perineural space26 (Fig. 14-1). The extrinsic circulation is under adrenergic control and thus highly responsive to epinephrine-containing solutions.
Pathogenesis of Peripheral Nerve Injury
Peripheral nerve injury may occur through a variety of mechanisms, with patient, surgical, and anesthetic risk factors all being major contributors (Box 14-1). However, regardless of the etiology, the pathogenesis of peripheral nerve injury remains fairly constant. Once injury occurs, those components distal to the site of injury will degenerate and be destroyed by phagocytosis. This process—termed secondary or Wallerian degeneration—may require up to 4 weeks for completion. However, morphologic changes will begin to occur within the first 72 hours. During this time period, the nerve may retain a variable degree of functionality while distal axonal segments begin to shrink secondary to fragmentation and fluid loss. As a result, these components assume a more oval or globular appearance, which promotes subsequent phagocytosis. At approximately 1 week postinjury, macrophages have reached the site of injury in greater numbers—clearing nearly all debris within 2 to 4 weeks. Schwann cell division by mitosis occurs simultaneously with this process, increasing the number of cells available to “fill” the area once occupied by the distal axon and myelin sheath.27
The reaction proximal to the site of injury is referred to as primary or retrograde degeneration. The time required for this process to occur is somewhat dependent upon sensory and motor segments, as well as the on the relative size and myelination of nerve fibers. Retrograde degeneration proceeds for at least one internodal space—being highly dependent upon the degree of proximal insult. Morphologic changes in the parent cell body vary to some degree with the type of cell and its proximity to the site of injury. The more proximal the site of injury, the more pronounced the changes within the axonal cell body. Histologically, severe injury may be evident by chromatolysis, or the swelling of the cytoplasm with eccentric displacement of the cell’s nucleus. If present, this will generally occur within the first 5 to 7 days, with death or evidence of recovery beginning over the next 4 to 6 weeks. With recovery, the edema begins to subside, the nucleus once again migrates to the center of the cell, and Nissl substance begins to reaccumulate.27
Axonal regeneration may begin within the first 24 hours after injury. During this process, endoneurial tubes and their associated Schwann cells will reorganize distal to the site of injury at the most proximal point of retrograde degeneration. In this location, the endoneurial tubes are optimally positioned to accept regenerating nerve sprouts from the axonal stump. All nerve sprouts are initially unmyelinated, whether they arise from myelinated or unmyelinated nerve fibers. If the endoneurial tube has been uninterrupted during the injury, axonal sprouts may readily pass along their former courses, innervating endorgans over the course of several weeks to months. However, if the injury was severe enough to disrupt the endoneurial tube structure and its associated Schwann cells, multiple axonal sprouts will begin to migrate aimlessly throughout the damaged area into the epineurial, perineurial, or adjacent regions to form a stump neuroma.27 If this occurs, it is unlikely that subsequent reinnervation of distal endorgans will occur.
Classification of Peripheral Nerve Injuries
The classification of peripheral nerve injuries was initially introduced by Seddon et al.28 in 1943. Although this classification was generally accepted, it was rarely used because of its poor association with clinical usefulness. However, many of the terms currently used to describe the severity of nerve injury were first introduced by Seddon (Box 14-3).
Following the description of Seddon, Sunderland29 developed one of the most widely used and recognized classifications of peripheral nerve injuries. The classification is clinically relevant, with each degree of injury suggesting a greater anatomic disruption—and thus an increasingly altered prognosis. A modified summary of the Sunderland Classification System is provided in Table 14-3.
BOX 14-3 Terms Used to Classify Peripheral Nerve Injury
Neuropraxia: A minor contusion or compression of a peripheral nerve with the preservation of the axis cylinder. Minor edema within the cell body or focal disruption of the myelin sheath may result in a transient disruption of nerve conduction signaling. However, this generally recovers and resolves within days to weeks.
Axonotmesis: A more significant injury with breakdown of the axon and subsequent Wallerian degeneration. However, endoneurial tube structures and their associated Schwann cells are well preserved, thus allowing spontaneous regeneration and good functional recovery over time.
Neurotmesis: A severe injury secondary to an avulsion or crush injury with complete axonal transection. The axon, endoneurial tube structures, and associated Schwann cells are completely disrupted. The perineurium and epineurium are also disrupted to varying degrees. In general, significant functional recovery is unlikely.
TABLE 14.3 Sunderland Classification of Nerve Injury
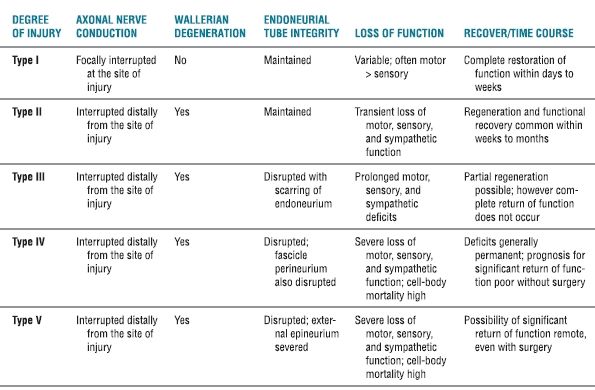
Adapted from Sunderland S. A classification of peripheral nerve injuries producing loss of function. Brain 1951;74:491–516.
 RISK FACTORS
RISK FACTORS
Peripheral nerve injury following regional anesthetic techniques may be the result of several contributing factors (Box 14-1). Patient-, surgical-, and anesthetic-related risk factors have all been identified as potential etiologies.30,31
Patient Risk Factors
Patient risk factors most commonly associated with perioperative nerve injury include the male gender,32 increasing age,32 the extremes of body habitus,32 diabetes mellitus,8,32–37 and preexisting neurologic deficits (from several etiologies).8,32–34,36,38–42 Preexisting neurologic deficits may include conditions such as diabetic neuropathy, chemotherapy-induced peripheral neuropathy, spinal stenosis, compressive radiculopathy, or multiple sclerosis (MS). Although MS is generally considered a disease of the central nervous system, patients with MS may be at increased risk of peripheral nerve lesions as well.43 Many clinicians are unaware that subclinical neural compromise may be present within the PNS of patients with MS.44 In fact, subclinical sensorimotor deficits have been identified in 45%45 to 78%46 of MS patients, with up to 43% having abnormalities in more than one peripheral nerve distribution. Finally, tobacco use8,22,33,47–49 and hypertension8 have also been implicated as contributors to peripheral nerve injury. Welch et al.8 speculate that chronic hypertension may render nerves more susceptible to injury because of alterations in blood flow (i.e., microvascular changes) or a greater propensity for hemodynamic instability (i.e., hypoperfusion) during the perioperative period.
Regardless of the underlying etiology, the presence of chronic neural compromise secondary to mechanical (e.g., compression), ischemic (e.g., peripheral vascular disease), toxic (e.g., cisplatin chemotherapy), or metabolic (e.g., diabetes mellitus) derangements may theoretically place patients at increased risk of further neurologic injury.50–52 Upton and McComas50 were the first to describe the “Double-Crush” phenomenon, which suggests that patients with preexisting neural compromise may be more susceptible to injury at another site when exposed to a secondary insult (Fig. 14-2). Secondary insults may include a variety of concomitant patient, surgical, or anesthetic risk factors. Osterman51 emphasized that not only are two low-grade insults along a peripheral nerve trunk worse than a single site, but that the damage of the dual injury far exceeds the expected additive damage caused by each isolated insult. It may be further postulated that the second “insult” need not be along the peripheral nerve trunk itself, but rather at any point along the neural transmission pathway. Therefore, the performance of neuraxial—as well as peripheral—techniques in patients with preexisting neurologic disorders may theoretically place them at increased risk of a “Double-Crush” phenomenon.
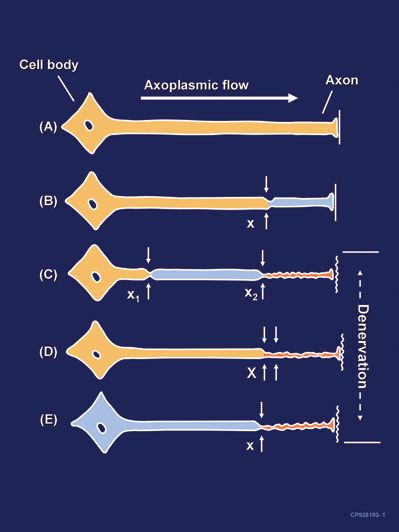
FIGURE 14-2. Neural lesions resulting in denervation. Axoplasmic flow is indicated by the degree of shading. Complete loss of axoplasmic flow results in denervation (C, D, E). A: Normal neuron. B: Mild neuronal injury at a single site (x) is insufficient to cause denervation distal to the insult. C: Mild neuronal injury at two separate sites (x1 and x2) may cause distal denervation (i.e., “Double Crush”). D: Severe neuronal injury at a single site (X) may also cause distal denervation. E: Axon with a diffuse, preexisting underlying disease process (toxic, metabolic, ischemic) may have impaired axonal flow throughout the neuron which may or may not be symptomatic, but predisposes the axon to distal denervation following a single minor neural insult at x (i.e., “Double Crush”). (By permission of Mayo Foundation for Medical Education and Research.)
Surgical Risk Factors
Surgical risk factors include direct intraoperative trauma or stretch,30,53–55 vascular compromise,56,57 perioperative inflammation or infection,12,22 hematoma formation,58 prolonged tourniquet ischemia,9,59 or improperly applied casts or dressings. Horlocker et al.12 examined the etiology of perioperative nerve injuries in 607 patients undergoing 1,614 blocks for upper extremity surgery. Surgical variables were believed to be the etiology in 55 (88.7%) of 62 neurologic complications identified. Direct surgical trauma or stretch occurred in 40 (73%) cases, inflammation or infection in 6 (11%) cases, hematoma or vascular compromise in 4 (7%) cases, cast irritation in 3 (5%) cases, and tourniquet ischemia in 2 (4%) cases. Interestingly, all complications involving motor deficits had a surgical cause. Fourteen patients (25%) required subsequent surgical intervention to restore nerve function.12 Barrington et al.11 have also demonstrated that the majority (90%) of postoperative neurologic deficits are from nonanesthesia-related etiologies.
Jacob et al.9 examined patient, surgical, and anesthetic risk factors associated with perioperative nerve injury in 12,329 patients undergoing total knee arthroplasty. Bilateral surgical procedures and total tourniquet time were surgical factors associated with an increased risk of nerve injury. For every 30-minute increment in tourniquet time, the risk of nerve injury increased by 28%.9 Previous investigators have also demonstrated that the likelihood of postoperative neurologic dysfunction increases with prolonged tourniquet inflation times and that reperfusion intervals only modestly decrease the risk of nerve injury.59
Intraoperative mechanical forces such as surgical retraction, stretch, compression, and contusion are believed to be major contributors to perioperative nerve injury. However, when the clinical features of peripheral nerve injury cannot be explained by surgical trauma, intraoperative positioning, or regional anesthetic techniques, alternative etiologies must be considered. One such condition is postsurgical inflammatory neuropathy—an uncommon and under-appreciated cause of perioperative neurologic dysfunction. Although poorly understood, the condition is believed to be an idiopathic immune-mediated response to a physiologic stress such as a surgical procedure, a vaccination, or an infectious process.22 The neuropathy may present with focal, multifocal, or diffuse neurologic deficits in the setting of negative radiographic imaging and normal ancillary serum testing. The onset of neurologic deficits following the surgical procedure is often delayed (i.e., the deficits are not immediately apparent after surgery) or in an anatomic distribution remote from the surgical site or regional anesthetic technique. Risk factors for postsurgical inflammatory neuropathy include diabetes mellitus, malignancy, systemic infection, and a history of tobacco use.22 Other potential triggers include recent blood transfusion or volatile anesthetic use, both of which are known to cause immune suppression.
Anesthetic Risk Factors
Regional anesthetic factors that may contribute directly or indirectly to perioperative nerve injury include needle- or catheter-induced mechanical trauma, ischemic nerve injury secondary to vasoconstrictors or neural edema, or chemical injury that may result from direct local anesthetic neurotoxicity.30,60
Mechanical Trauma: The Role of Needle Injury
Several authors have investigated the role of mechanical trauma, including the elicitation of paresthesias and the role of needle type, on peripheral nerve injury.12,20,21,39,61–67 Animal models have implicated needle gauge, type (long vs. short bevel), and bevel configuration as contributing to nerve injury.61,65 Selander et al.65 examined the role of needle trauma by piercing rabbit sciatic nerves, either isolated (in vitro) or in situ, using two different needle types. Both long- (14 degrees) and short-bevel (45 degrees) needles were examined. They found that neuronal injury occurred more frequently with long-beveled needles when compared to short-beveled ones (90% vs. 53%) in isolated (in vitro) nerve preparations. More pronounced fascicular sliding occurred in situ, resulting in a lower frequency of nerve injury with both needle types (long-bevel 47% vs. short-bevel 10%). While the overall frequency of nerve injury occurred less often when short-beveled needles were used, those injuries elicited were often as severe, if not more severe, than those seen with long-beveled needle injuries. Selander concluded that short-beveled needles significantly reduce the risk of fascicular injury and should therefore be used in clinical practice when performing PNB.
Scientific data from anatomic dissections and histologic findings may further support the notion that traumatic nerve injuries occur less often with short-beveled needles. Previous studies have shown that the much of the cross-sectional area of a peripheral nerve consists of connective tissue. This anatomic finding is particularly true with more distal peripheral nerves.68–70 Because short-bevel needles are more likely to penetrate loose connective tissue than nerve fascicles protected by a dense fibrous perineural membrane,71 one may speculate that short-bevel needles are “protective” against severe intrafascicular injury. This phenomenon may explain why select patients who receive an intraneural (but not intrafascicular) injection do not develop postoperative neurologic deficits.72,73 In contrast to short-bevel needles, sharp needles have been found to penetrate cryopreserved cadaveric human sciatic nerves 3.2% of the time.71
Rice and McMahon61 have also investigated the role of needle size and configuration on peripheral nerve injury in a rat model. Unlike Selander, who reported histologic findings within 2 hours of injury, Rice and McMahon examined the histologic, functional, and behavioral effects of nerve impalement 28 days following the traumatic event. They found that long-beveled needles in the parallel configuration produced less intraneural damage than transverse long-beveled or short-beveled needles immediately after injury and at 7 days. However, by 28 days, all injuries caused by long-beveled needles (parallel and transverse) were resolving, while those induced by short-beveled needles continued to display evidence of severe injury. Overall, nerve injury scores were also significantly lower for long-beveled needles 4 weeks following injury when compared to short bevels (Fig. 14-3). The authors concluded that when nerve fascicles are impaled by commercially available injection needles, lesions occur less frequently, are less severe, and are more rapidly repaired if they are induced by a long-beveled needle.
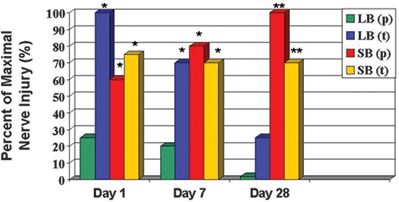
FIGURE 14-3. Percent of maximal rat sciatic nerve injury as a function of time and needle bevel type and orientation. Nerve injury is determined by the cumulative score of three graded components: intraneuronal disruption (graded 0–5), axonal degeneration (graded yes/no), and disorganized fiber regeneration (graded yes/no). Nerve lesions induced by short-bevel needles are more severe and take longer to repair than those induced by long-bevel needles. Nerve injury induced by short-bevel needles was often associated with persisting signs of injury 28 days after injury. LB(p), long-bevel needle in parallel configuration to nerve fibers; LB(t), long-bevel needle in transverse configuration to nerve fibers; SB(p), short-bevel needle in parallel configuration to nerve fibers; SB(t), short-bevel needle in transverse configuration to nerve fibers. *p < 0.05 versus LB(p) **p < 0.05 versus LB(p) and LB(t) (Reprinted with permission of Oxford University Press/British Journal of Anaesthesia.© The Board of Management and Trustees of the British Journal of Anaesthesia.)
While these results appear to disagree with those of Selander’s, two fundamental differences exist between the two studies. First, Selander et al. examined the immediate (2-hour) frequency of injury to the perineural membrane, which surrounds nerve fascicles, after nerve trunk impalement. Rice and McMahon examined the frequency, severity, and long-term (28 days) consequences of injury to fascicular contents after in vivo impalement of nerve fascicles. Second, different animal models were used by the two authors. Selander et al. used a rabbit sciatic nerve preparation, which is multifasciculated and often results in the sliding of nerve fascicles away from needle tips. They discussed the significance of this and postulated that it may have been a factor in the differing frequency of fascicular injury after the use of short- versus long-beveled needles. In contrast, Rice and McMahon used the rat sciatic nerve which consists of a single, large nerve fascicle at the level of the mid-thigh. Therefore, no fascicular sliding was possible, and neural (fascicular) penetration was ensured in all cases of impalement.
In summary, it appears from Selander’s work that the likelihood of a traumatic insult may occur less frequently with short-beveled needles as a result of fascicular sliding that may occur within nerve trunks. However, when fascicular impalement does occur, both studies are in agreement that nerve injury may be more severe with short-bevel needles. In addition, Rice has demonstrated that neural repair may be accelerated and more organized with long-beveled injuries. Therefore, long-term consequences may be less of a concern when long-beveled needles are used. Furthermore, it has been suggested that sharper needles may be more comfortable for patients.74 However, it must be emphasized that there are no confirmatory human studies to support—nor refute—the ability of various needle types and bevel configurations to impale human nerves within the clinical setting. Further clinical study is necessary before definitive recommendations can be made with regard to the optimal type and configuration of needles used during PNB.
Mechanical Trauma: The Role of Paresthesias
Traditionally, anesthesiologists would intentionally elicit a paresthesia during the performance of PNB to reliably localize neural structures. This may have originated, in part, from the long-established dictum “no paresthesia, no anesthesia.”75 Although the elicitation of a paresthesia may represent direct needle trauma, and theoretically increase the risk of neurologic injury, there are no prospective, randomized clinical studies that are able to definitively support nor refute these claims.20,39,63,66,67,76,77 Selander et al.20 performed one of the early prospective investigations examining the role of paresthesias and nerve injury. They reported a higher incidence of postoperative neurologic complications in patients where a paresthesia was intentionally sought during axillary blockade (2.8%) compared to those undergoing a perivascular technique (0.8%). While the difference was not found to be statistically significant, it should be noted that unintentional paresthesias were elicited and injected upon in patients within the perivascular group who experienced postoperative nerve injury. Winnie78 argues that these results do in fact become statistically significant when groups are separated into those who actually experienced paresthesias versus those who did not. Overall, 40% of patients within the perivascular group reported unintentional paresthesias during the procedure, demonstrating the difficulty with standardization of technique and analysis of nerve injury. Postoperative neurologic deficits ranged from slight hypersensitivity to severe paresis and persisted from 2 weeks to >1 year. Interestingly, 67% of patients with injuries lasting >52 weeks had supplementation of their block following the initial regional technique. Theoretically, supplemental injections may significantly increase the risk of neural injury because of the absence of warning signs such as paresthesias when probing into a partially anesthetized plexus.
Urban and Urquhart39 also performed a prospective investigation utilizing a variety of regional anesthetic approaches, including transarterial, paresthesia, and nerve stimulator techniques during brachial plexus blockade. The incidence of persistent postoperative paresthesias was 9% on the first day after surgery and 3% at 2 weeks in patients undergoing interscalene blockade. The majority of complaints consisted of hypoesthesia in the hand and resolved within 6 weeks in all but one patient (0.4%). The incidence of paresthesias at 2 weeks was significantly higher in those patients receiving bupivacaine compared to mepivacaine or lidocaine (p = 0.013). However, this observation was attributed to poor arm positioning during prolonged postoperative sensory anesthesia and not to local anesthetic toxicity. Patients undergoing axillary blockade had even higher complication rates. Paresthesias persisted into the first postoperative day in 19% of patients. These were not associated with the type of local anesthetic, the number of needle advances, anesthetic technique (paresthesia vs. transarterial), or the duration of tourniquet inflation. However, there was a significant increase in the incidence of acute postoperative dysesthesias in those patients who had preoperative neurologic symptoms within the extremity (p = 0.02). Only 5% of patients continued to experience new postoperative paresthesias at 2 weeks. Symptoms were confined to numbness and tingling within the fingers and forearm hyperesthesia. Once again, all but one patient (0.4%) had resolution of their symptoms by 4 weeks. Interestingly, a large percentage of patients in both groups (54% interscalene; 18% axillary) experienced a paresthesia on injection. In the axillary group, this occurred in many cases without an initial paresthesia during needle placement.
Investigations in which local anesthetic was not injected onto paresthesias have resulted in significantly lower neurologic complication rates than those described above.63,67 Selander63 examined the role of axillary catheter placement in providing prolonged or continuous brachial plexus anesthesia. During catheter placement, an unintentional paresthesia was obtained in 39% of patients. However, local anesthetic was never injected at the time of paresthesia elicitation. Following catheter placement and negative aspiration, local anesthetic was injected through the cannula with no report of pain or pressure paresthesias. Surprisingly, there were no neurologic sequelae (0%) postoperatively. Similarly, Stan et al.67 report only a 0.2% incidence of neurologic complications in 996 patients undergoing transarterial axillary blockade. If an unintentional paresthesia was elicited during the performance of the block, the needle was redirected toward the artery with no injection of local anesthetic. Of the 119 (12%) patients in whom an unintentional paresthesia was elicited during the procedure, none reported a neurologic injury postoperatively. It is suspected that the two patients (0.2%) who did experience new sensory paresthesias postoperatively suffered unintentional mechanical trauma or intraneuronal injection during subsequent block supplementation.
Investigations such as those cited above appear to associate regional anesthesia-induced perioperative nerve injury with the injection of local anesthetic onto an elicited paresthesia. However, it has been argued that many of the studies condemning paresthesias have been performed in animal models in which nerve impalement occurs under direct vision.79 Moore79 suggests that a properly elicited paresthesia does not indicate that: (i) the bevel of the needle has punctured the epineurium; (ii) the nerve has been impaled; (iii) the neural fibers have been cut; or (iv) an intraneural injection will occur. He further emphasizes that no statistically significant clinical data have been published that demonstrate properly elicited paresthesias during regional blockade result in the temporary or permanent loss of neural function. Investigations by Winchell and Wolfe 66 and Pearce et al.76 appear to support Moore’s claim. Winchell and Wolfe66 report only a 0.36% incidence of postoperative neurologic injury in 854 patients undergoing brachial plexus blockade. Of these, 835 (98%) experienced a paresthesia with subsequent injection of local anesthetic during the procedure. All postoperative neurologic sequelae involved diminished sensation within the distal extremity and had resolved within 7 months. Pearce et al.76 prospectively examined the complication rates of four techniques (transarterial, paresthesia, transvenous, and tethering) used to identify the axillary neurovascular plexus. A paresthesia was elicited in 24% of patients during needle placement, and a pressure paresthesia occurred in 58% during local anesthetic injection. Overall, complications included mild acute local anesthetic toxicity (3.5%), axillary tenderness and bruising (9%), axillary hematoma (3%), and postoperative dysesthesias (12.5%). Interestingly, patients in whom the brachial plexus was identified using the transarterial technique had significantly more dysesthesias on the day following surgery when compared to all other groups. The authors postulate that subclinical hematoma formation, not paresthesias, may be contributing to the development of transient postoperative neurologic symptoms (PONSs).
Ischemic Injury: The Role of Epinephrine and Neural Edema
Epinephrine is a common adjuvant often added to local anesthetics during PNB. In a concentration of 1:200,000, epinephrine not only prolongs the duration of local anesthetic blockade, but also reduces the vascular uptake within the anesthetized area.80 This adrenergic effect avoids the rapid redistribution of these drugs to the systemic circulation where they may cause potentially devastating toxic reactions. As a result, many authorities have recommended its use in procedures of neural blockade in tissues that do not have terminal arteries.
However, recent investigations have focused on the role of epinephrine and neural blood flow (NBF) in the pathogenesis of nerve fiber injury.26,81–83 It has long been recognized that the functional integrity of peripheral nerves is highly dependent upon its microcirculation.25 The blood supply to peripheral nerves consists of two sources: (i) an intrinsic supply of exchange vessels within the endoneurium and (ii) an extrinsic supply of larger, nonnutritive feeding vessels (Fig. 14-1). This extrinsic vasculature runs longitudinally along the nerve within the perineural space and crosses the perineurium to anastomose with the intrinsic circulation.26 The epineurial circulation is a critical component of the overall neural circulation. Stripping the epineurium of this extrinsic vascular supply results in a 50% reduction in NBF and subsequent demyelination of intrinsic nerve fibers.84 It is also this extrinsic circulation that is under adrenergic control and thus highly responsive to epinephrine-containing solutions.85
Myers and Heckman26 examined the role of lidocaine, with or without epinephrine, in reducing NBF within rat sciatic nerve preparations. The topical application of 2% lidocaine resulted in a 39% reduction in NBF when compared to saline controls. The addition of epinephrine (1:200,000) to the local anesthetic resulted in an even greater reduction in NBF than 2% lidocaine alone (78% vs. 39%). Both values were significantly less than baseline and saline control values (Fig. 14-4). Differences between the 2% lidocaine and 2% lidocaine/epinephrine solutions were also statistically significant. As a result, it has been postulated that epinephrine may induce severe neural ischemia, resulting in potentially irreversible pathologic damage to nerve fibers and their supporting cellular structures. However, this risk of nerve injury may be limited to only those individuals with underlying toxic (e.g., chemotherapy) or metabolic (e.g., diabetes) neuropathies (Fig. 14-2E).86 Under these clinical conditions, it may be prudent to minimize the concentration of epinephrine used during peripheral blockade. In contrast, the use of epinephrine and associated neural ischemia does not appear to be a concern in patients with normal underlying vascular and neuroanatomy.
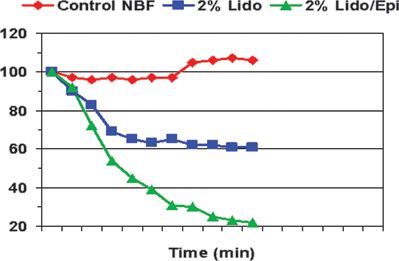
FIGURE 14-4. The effect of lidocaine on nerve blood flow. Effects of lidocaine 2% and lidocaine 2% with epinephrine on rat sciatic NBF. (Reprinted from Myers RR, Heckman HM. Effects of local anesthesia on nerve blood flow: studies using lidocaine with and without epinephrine. Anesthesiology 1989;71:757–762, with permission.)



