Fig. 51.1
One half of a cross-section through the spinal cord, showing the innervation. As described in the text, the sinuvertebral nerve supplies the anulus fibrosis and the posterior longitudinal ligament—two structures that play an important role in the development of back pain. (1) Facet, (2) posterior primary ramus, (3) anterior ramus, (4) sinuvertebral nerve, (5) sympathetic, (6) root, (7) nucleus, (8) anulus (With permission from Danilo Jankovic)
Kusslich et al. concluded that “Sciatica can only be caused by direct pressure or traction on an inflamed, stretched, or compressed nerve root. No other tissue in the spine is able to trigger pain in the leg.”
However, nerve roots are exposed not only to mechanical effects but also to proinflammatory material from degenerated intervertebral disks or facet joints [14].
lower level for Pressure (Fig. 51.2)
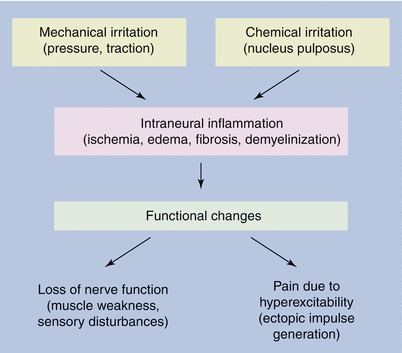
Fig. 51.2
Mechanical and chemical stimulants trigger the development of neuraxial pain and radiculopathy, as well as cervicogenic headache [12]
The effects of pressure exerted on the nerves depend on whether the pressure is high or low. High pressure can produce direct mechanical effects on the nerve tissue and can distort the nerve fibers, shift the nodes of Ranvier, or press in the paranodal myelin sheath. Lower pressures lead to tissue changes caused by a reduction in the blood supply to the nerve tissue. In animal experiments, it has been found that when inflation of a balloon attached to the spinal cord makes the pressure on the cauda equina equivalent to arterial pressure, blood flow in the cauda equina is interrupted [14]. Even a pressure of 5–10 mmHg interrupts venous blood flow in some small veins, while a pressure of 10 mmHg reduces the transport of nutrients to the nerve roots by 20–30 %. Compression can also cause changes in the permeability or transmural pressure conditions in the endoneurial capillaries in the nerve roots and can lead to edema formation (in the animal experiment, e.g., this occurred after 2 min of compression at 50 mmHg).
Intraneural edema due to chronic nerve injury is associated with the development of neural fibrosis, which may contribute to the very slow rate of symptomatic improvement observed in some patients with nerve compression.
Compression of spinal nerves at 10 mmHg for 2 h by two adjacent balloons (to simulate the clinical conditions occurring in multiple nerve compression) reduced neural conduction and led to a reduction in the recorded amplitudes of action potentials by ca. 65 %. By contrast, compression by a single balloon at 50 mmHg for 2 h did not alter the amplitude of the action potentials. A pressure of 10 mmHg (with incomplete blockage of small veins) only appears to be capable of causing changes in nerve function if the spinal nerve roots are compressed into two segments [14]. Intervertebral disk prolapses or protrusions can cause higher levels of compression pressure than central spinal stenosis.
Chemical Irritation (Fig. 51.2)
Some chemical substances have been identified in the nucleus pulposus that can lead to irritation of neighboring structures if tears in the anulus fibrosus lead to them being released into the vertebral canal. These substances, which can cause inflammation of nerve roots and meninges, include lactic acid, glycoprotein, cytokines, and histamine. In addition, it is considered theoretically possible that components of the nucleus pulposus can act as foreign proteins and trigger an autoimmune reaction. This type of chemically caused irritation can arise without any compression by an intervertebral disk.
Structural Changes
Intervertebral disk anomalies can manifest as degenerative changes, protrusion, or herniation. Constriction of the intervertebral space due to disk injury is often associated with osteophyte formation and arthrosis of the facet joints, which can lead to increased pressure on the spinal nerves. Stretching of the posterior longitudinal ligament by protruding intervertebral disks initially leads to localized back pain, while more severe protrusions also cause pressure on neighboring nerve roots and can lead to radicular pain.
Theoretical Considerations
In patients with chronic neuraxial pain and/or radiculopathy or cervicogenic headache, one or more of the following pathological changes may be present (Fig 51.2):
Inflammation
Edema
Fibrosis
Venous engorgement
Mechanical pressure on:
Posterior longitudinal ligament
Anulus fibrosus
Spinal nerve
Reduced or absent nutrient supply to the spinal nerves or nerve roots
Central sensitization
The inflammatory tissue changes can activate nociceptors or axons that conduct nociceptive information to the CNS. Equally, owing to inflammation, nociceptors or nociceptive axons may react more sensitively to mechanical stimuli. This type of mechanical irritation may be triggered either by pressure stress, as described above, or may be caused by movement-dependent stretching due to entrapment of spinal nerves or nerve roots by fibrous tissue.
Most experience with neuroplasty has been gathered in the treatment of chronic pain conditions. The patients probably have both peripheral and central changes (e.g., central sensitization) contributing jointly to the chronic pain condition.
It is therefore theoretically justifiable to treat back pain with or without radiculopathy by local administration of drugs. Possible forms of treatment include:
Anti-inflammatory drugs (e.g., corticosteroids).
Drugs that reduce edema formation (e.g., hypertonic saline 10 %, hyaluronidase, and corticosteroids).
Local anesthetics to block the nerve fibers that conduct pain information to the CNS (hypertonic saline also has a local anesthetic effect).
Addition of hyaluronidase to remove scar tissue. This makes it possible for the drug being used to reach the target tissue.
Figure 51.3 shows the selection criteria for patients who are candidates for percutaneous epidural neuroplasty; noninvasive, conservative treatment methods should be attempted first. Our technique is described in Table 51.1. Table 51.2 lists the most frequently used injection solutions.
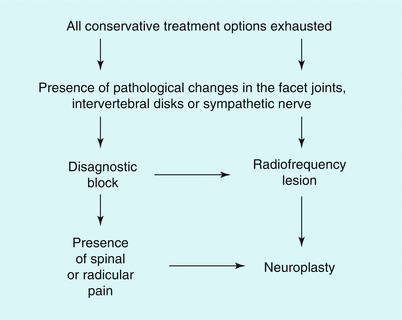

Fig. 51.3
Algorithm for the treatment of neuraxial pain and radiculopathy, as well as cervicogenic headache. Neuroplasty is considered when conservative forms of treatment have proved to be ineffective and the appropriate diagnosis is confirmed
Table 51.1
Overview of neuroplasty
In the operating room: |
1. Placement of the epidural needle |
2. Injection of contrast and imaging of its spread (epidurogram) |
3. If there is a filling defect in the area in which the pain is located, introduction of a Racz catheter into the filling defect (scar) |
4. Repeat contrast injection to ensure that the contrast is now also spreading in the area of the filling defect |
5. Injection of preservative-free saline, with or without hyaluronidase |
6. Injection of local anesthetic and corticosteroids |
7. Secure the catheter |
In the recovery room: |
8. 30 min after the injection of a mixture of steroids and local anesthetic, infusion of 10 mL hypertonic saline (10 %) over 30 min |
On the ward: |
9. On the following day, inject local anesthetic followed 30 min later by hypertonic saline (10 %) twice with a 6–8 h interval between injections |
10. The epidural catheter is removed after the final treatment |
Table 51.2
Injected solutions according to the spinal cord section (volume in mL) in the injection sequence
Solution | Cervical | Thoracic | Lumbar | Caudal |
|---|---|---|---|---|
Iohexol | 2–3 | 4–6 | 10 | 10 |
Iohexol | 1–2 | 2–3 | 3 | 3 |
Saline 0.9 % + 1,500 IU hyaluronidase | 4–6 | 6–8 | 10 | 10 |
Ropivacaine 0.2 % + corticosteroida (test dose) | 2 | 2 | 3 | 3 |
Ropivacaine 0.2 % + corticosteroida | 2–4 | 4–6 | 7 | 7 |
Hypertonic saline | 4–6 | 6–8 | 10 | 10 |
Saline 0.9 % | 2 | 2 | 2 | 2 |
Then, on each of the 2 following days: | ||||
Ropivacaine 0.2 % + corticosteroid (test dose) | 2 | 2 | 3 | 3 |
Ropivacaine 0.2 % | 4 | 6 | 7 | 7 |
Hypertonic saline | 4–6 | 6–8 | 10 | 10 |
Saline 0.9 % | 2 | 2 | 2 | 2 |
Techniques of Cervical Epidural Neuroplasty
Caudal Access Route
Procedure
Careful Information Discussion with the Patient Before the Block
Full prior information for the patient is mandatory. The patient should be informed about all of the potential complications that can occur during and after the procedure (e.g., epidural hematoma, epidural abscess, numbness in the extremities, rectal or bladder emptying disturbances, paralyses, infection, sexual dysfunction, shearing of the epidural catheter, etc.).
Materials
See Chap. 47 on epidural caudal anesthesia.
16-G or 15-G RX Coudé needles—fluoropolymer-coated epidural catheter made of stainless steel with a spiral tip TunL Kath or TunL-XL (stiffer); Epimed International, Inc., Irving, Texas, USA).
Preparations
See chapter on epidural caudal anesthesia.
Intravenous access is required in order to treat potential adverse events (e.g., total spinal anesthesia, subdural injection, intravascular injection, etc.), as well as to administer analgesia and sedation during the procedure and antibiotics postoperatively.
Analgesia and sedation are recommended before the procedure (e.g., 1–2 mg midazolam + 25–50 μg fentanyl), as the injection is usually painful in patients with epidural adhesions. The injection pain is probably caused by stretching of the nerve roots affected, and it spreads in the corresponding cutaneous innervation area. The patient should not receive deep sedation. The patient needs to be capable of cooperating during the procedure to ensure that any signs of spinal cord compression are not overlooked. (The patient has to be able to move the extremity affected and report any weakness or paralysis during the procedure.)
All procedures are conducted under fluoroscopic guidance, using a C-arm with a storage function in pulse mode (reduced radiation exposure). Fluoroscopic guidance optimizes the results of the procedure (correct needle positioning, easier identification of the defect, ability to check the spread of the contrast, and correct positioning of the catheter). The usual protective measures for staff are obligatory.
Selection of Drugs
Radiographic Contrast Media
To exclude inadvertent subarachnoid injection, a water-soluble, nonionic contrast medium, i.e., iohexol 240, is used. In our experience, the presence of epidural adhesions increases the risk of subarachnoid injections. Subarachnoid injection of a contrast medium that is not water soluble can lead to serious complications (spinal cord irritation, spinal cramp or clonus, arachnoiditis, paralysis, and death).
Local Anesthetic
For example, 0.2 % ropivacaine.
Corticosteroids
The choice of the corticosteroid to be used (Table 51.2) mainly depends on which agents are available. Long-acting steroid emulsions have a particle size of ca. 20 μm and it is therefore not possible to inject them through bacterial filters.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








