Chapter 16 Pediatric Intensive Care in Developing Countries
In the year 2007, 136 million children were born in the world. Of these children, 9.2 million died before age 5 years, with 98% of the deaths occurring in developing countries.1 If the entire world had an mortality rate for children younger than 5 years equal to that of the developed world, only 0.8 million deaths would occur in this age group each year.1 Therefore, of the 9.2 million deaths in children younger than 5 years that occur each year, 8.4 million are preventable.
Why Lower Child Mortality Rates?
If too many people already exist in the world, is it sensible to try to reduce child mortality rates? First, this question is always asked about other people’s children. Second, reducing child mortality rates is important both for humanitarian reasons and to enable lower birth rates.2 Governments of poor countries are not able to provide old-age or sickness benefits, so having children who survive to adulthood is crucially important to provide security. To increase their chances of having their children survive when mortality rates are high, people need to have many children. A vicious circle is created because high birth rates perpetuate poverty so that governments cannot provide social security, people need children who survive to adulthood, and birth rates remain high. Therefore reducing child mortality rates is a necessary (but not sufficient) condition for reducing birth rates and slowing the growth of the world’s population.2
Expenditure on Health
In 2006, 1.3 billion people lived in the world’s 43 poorest countries (with an annual gross national income of less than U.S. $976 per capita), with an average total annual expenditure on health of only U.S. $23 per capita.3 In the world’s 66 high-income countries, annual expenditure on health averaged U.S. $4033 per capita, which is 175 times the amount available in low-income countries.
Child Mortality, Infections, and Intensive Care
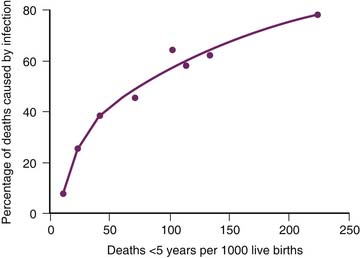
Most of the unnecessary child deaths in developing countries are caused by infectious diseases, and most of these deaths can be prevented by immunization and basic primary health care. Figure 16-1 shows the relationship between the mortality rate among children younger than 5 years and the percentage of deaths caused by infection.4 When the mortality rate among children younger than 5 years is less than 20 per 1000 live births, few deaths are caused by infections, and intensive care can make an important contribution to reduce mortality from noninfectious causes such as congenital heart disease and trauma. As the mortality rate among those younger than 5 years increases from 20 to 30 per 1000, the proportion of deaths caused by infections increases rapidly, and the role of intensive care becomes less clear. When the mortality rate among children younger than 5 years is greater than 30 per 1000, many deaths are caused by infections, and a high proportion of these deaths can be prevented by immunization and primary health care, which are far less expensive than intensive care.
Role of Intensive Care
The main argument against providing intensive care in areas with a high mortality rate is that intensive care diverts scarce resources away from far more effective low-cost interventions such as immunization and primary health care. Skilled staff, in particular, are in short supply in many developing countries; if they are used to provide curative services in urban hospitals, the rural poor are at grave risk of being neglected. Strong arguments exist for providing basic public and primary health care to all children rather than intensive care to a small proportion of children.5 In addition, intubation and ventilation are difficult to do well. Done poorly, they may actually increase the incidence of mortality. For example, a study in six pediatric ICUs in Mexico and Ecuador found that endotracheal intubation and central venous cannulation were associated with an increased incidence of mortality in low-risk admissions.6
Ethical Dilemma
This dilemma cannot be resolved while extreme poverty persists in developing countries. Unfortunately, rich countries are doing even less to help now than in the past. Overseas development aid from members of the Organisation of Economic Co-operation and Development amounted to only 0.30% of their gross domestic product in 2007, which was well short of the United Nations’ target of 0.70%.7 Even worse, Organisation of Economic Co-operation and Development member countries now spend more than U.S. $1 billion every day on agricultural subsidies, which is more than six times the amount given in aid and seriously undermines primary producers in developing countries. If developing countries could increase their export share by just 5%, it would generate U.S. $350 billion per year, which is seven times more than the total amount they receive in aid.8,9
Causes of Death
More than 98% of all child deaths occur in developing countries, and 8.4 million of the 9.2 million deaths in children younger than 5 years are preventable.1 Table 16-1 shows that most of the deaths are caused by infectious diseases, particularly pneumonia, diarrhea, neonatal sepsis, and malaria, with malnutrition an important contributing factor.10,11 Table 16-2 shows that the major pathogens are Streptococcus pneumoniae, measles, Haemophilus influenzae, rotavirus, malaria, human immunodeficiency virus (HIV), and respiratory syncytial virus.12 The following sections of this chapter discuss individual diseases that are common causes of mortality in children in developing countries, starting with the diseases that cause the most deaths (see Table 16-1). On rare occasions, children with these diseases require intensive care in developed countries. The suggested treatments assume that the child is in a hospital that can deliver a high standard of intensive care. Other hospitals should follow the World Health Organization (WHO) guidelines for the care of children in developing countries.13–16
Table 16–1 Causes of Death in Children Younger than 5 Years in 2005
| Cause | No. of Deaths (in Millions) |
|---|---|
| Pneumonia | 2.0 |
| Diarrhea | 1.8 |
| Prematurity | 1.1 |
| Neonatal sepsis | 1.0 |
| Birth asphyxia | 0.9 |
| Malaria | 0.9 |
| Measles | 0.4 |
| Human immunodeficiency virus | 0.3 |
| Congenital abnormalities | 0.3 |
| Pertussis | 0.3 |
| Neonatal tetanus | 0.3 |
| Other | 1.3 |
| Total | 10.6 |
Data from World Health Organization: The World Health Report 2005: make every mother and child count, Geneva, 2005, World Health Organization.
Table 16–2 Approximate Annual Number of Deaths in Children Younger than 5 Years Caused by Individual Pathogens in 1999
| Pathogen | No. of Deaths (in Millions) |
|---|---|
| Pneumococcus | 1.2 |
| Measles | 1.1 |
| Haemophilus (a, b, c, d, e, f, nonserotypable) | 0.9 |
| Rotavirus | 0.8 |
| Malaria | 0.7 |
| Human immunodeficiency virus | 0.5 |
| Respiratory syncytial virus | 0.5 |
| Pertussis | 0.4 |
| Tetanus | 0.4 |
| Tuberculosis | 0.1 |
| Hepatitis B | <0.1 |
| Influenza virus | <0.1 |
| Meningococcus | <0.1 |
| Parainfluenza virus | <0.1 |
| Varicella | <0.1 |
| Total | 6.7 |
Data from Shann F, Steinhoff MC: Vaccines for children in rich and poor countries, Lancet 354(suppl ii):7, 1999.
Pneumonia
Pneumonia is the most common cause of death in children. It is the direct cause of 2.0 million deaths each year in children younger than 5 years. It also contributes to mortality from neonatal sepsis, measles, pertussis, and HIV, so pneumonia is an important contributory factor in approximately one third of all deaths in children younger than 5 years.17,18 A total of 156 million episodes of acute lower respiratory tract infection occur in children each year, 11 to 20 million of which are severe enough to require hospital admission.17
Fatal pneumonia in children usually is caused by S. pneumoniae or H. influenzae. The etiology of pneumonia can be determined accurately only by culture of lung aspirates from children with no antibiotic activity detectable in serum or urine.19 Most cases of pneumonia in children are caused by aspiration of bacteria from the nasopharynx, and mixed infections with S. pneumoniae, H. influenzae, and Moraxella catarrhalis are common.20 H. influenzae pneumonia often is caused by nonserotypable strains, as well as by type b and the other serotypes (a, c, d, e, f).21
Antibiotic Treatment
Children with pneumonia who are sick enough to require hospitalization usually should be treated with benzyl penicillin, and they should be treated with benzyl penicillin plus gentamicin if they have very severe pneumonia.15 The combination of benzyl penicillin and gentamicin has synergistic activity against many strains of S. pneumoniae and is always active against H. influenzae. Penicillin resistance is not an indication for the use of third-generation cephalosporins to treat pneumococcal pneumonia, because they are no more effective than penicillin alone,22 let alone penicillin plus gentamicin. However, if meningitis caused by a partially resistant strain is present, vancomycin or a third-generation cephalosporin (e.g., cefotaxime or ceftriaxone) should be used if available.
Staphylococcal pneumonia is suggested by a poor response to penicillin and gentamicin, pneumatoceles, pneumothorax, empyema, or associated soft tissue or joint infection. Cloxacillin (or oxacillin, flucloxacillin, or dicloxacillin) and gentamicin given intravenously are appropriate treatments.
Oxygen and Ventilation
Oxygen therapy may be lifesaving in patients with severe pneumonia.23,24 The most efficient means of administration is 1 to 2 L/min of humidified oxygen via an 8F nasopharyngeal catheter inserted 2 cm less than the distance from the side of the nose to the front of the ear. As well as providing oxygen, this method delivers low levels of CPAP.25 Care should be taken to (1) remove and clean the catheter every 12 hours, (2) verify that the catheter is not inserted too far (to avoid delivering oxygen into the esophagus), and (3) limit the flow to a maximum of 2 L/min (to avoid distending the stomach). A special low-flow oxygen flow meter with a scale of 0 to 2 or 0 to 3 L/min should be used.
If mechanical ventilation is not available, oxygenation and ventilation can be improved using mask or nasopharyngeal CPAP up to 12 cm H2O pressure. If endotracheal intubation and mechanical ventilation are available, a sensible plan is to start with low tidal volumes of 6 to 8 mL/kg, positive end-expiratory pressure 8 to 10 cm, inspiratory time 1 second with a rate of 20 to 30 per minute in an infant (assuming no bronchiolitis or asthma is present), and peak pressure less than 30 cm to minimize ventilator-associated lung injury (see Chapters 49 and 50). Failure of the right ventricle may occur as a result of pulmonary hypertension26; in this situation, nitric oxide, 5 to 10 ppm (or sildenafil) and high-frequency oscillatory ventilation may be helpful if available.
Fluid Therapy
Patients with pneumonia often exhibit increased secretion of antidiuretic hormone (syndrome of inappropriate antidiuretic hormone secretion),27,28 and thus children with pneumonia should not be given too much fluid. Hyponatremia usually is caused by excess water rather than sodium deficiency and should be treated by fluid restriction rather than administration of hypertonic saline solution. A small proportion of children with pneumonia have septic shock with capillary leak and hypovolemia, which may be exacerbated by positive pressure ventilation (causing severe hypoperfusion). A helpful procedure in children with this condition may be to insert a central venous catheter and give 10 mL/kg boluses of 4% to 5% albumin or 0.9% saline solution to achieve a central venous pressure of 10 to 12 mm Hg (see Chapters 29 and 103). In the absence of extensive skill and experience, femoral venous catheters are probably the safest to use. Large amounts of fluid initially may be needed to restore the intravascular volume, but thereafter fluid requirements often are only 30% to 40% of normal because the child may have high antidiuretic hormone concentrations.
Feeding
The blood glucose level should be monitored closely and the glucose infusion rate should be adjusted to prevent hypoglycemia. If necessary, 50% dextrose can be infused via the central venous catheter. Continuous small nasogastric feedings should be started from the time of admission as long as no cardiovascular compromise is evident. Full enteral feedings usually can be achieved within 24 to 48 hours. If gastric feedings are not tolerated, nasojejunal feedings may be successful if a nasojejunal tube can be placed (see Chapter 75).
Gastroenteritis
Gastroenteritis is the second most common cause of child mortality. It causes 2 million child deaths every year (see Table 16-1). Particular problems include shock, acid-base abnormalities, electrolyte abnormalities, and secondary bacterial infection.29 Diarrhea may be a symptom of other disease processes, including sepsis and metabolic abnormalities, so it should not be assumed to be caused by gastroenteritis.
The consequences of fluid loss depend on the rate and the amount of loss. In gastroenteritis, fluid moves from the intravascular space into the gut lumen. Depending on the relative rates of fluid loss and fluid replacement from the extracellular fluid space, patients may be in shock with no clinical signs of dehydration, dehydrated with no features of shock, or dehydrated and in shock. Clinical signs of dehydration occur when approximately 30 to 40 mL/kg of fluid is lost from the body (i.e., 3% to 4% dehydration).30
Hypovolemic shock requires immediate and vigorous replacement with isotonic IV fluids. Dehydration can be corrected over 2 or 3 days and usually can be adequately treated with oral ingestion of fluids. The management of dehydration is complicated by the relative inaccuracy of the clinical signs of dehydration (particularly in malnourished or obese children)30 and by the variable amount of ongoing stool losses (see Chapter 29). During rehydration, the serum sodium concentration should be measured every 4 hours and the rate of fluid and sodium administration adjusted accordingly.
Shock
Shock in patients with gastroenteritis usually is caused by hypovolemia resulting from fluid loss. However, there is a high incidence of bacterial sepsis in patients with severe gastroenteritis. The initial therapy for shock should include provision of oxygen and rapid IV administration of fluid with an electrolyte content similar to plasma (e.g., 0.9% sodium chloride). Shock should be corrected rapidly over 10 to 15 minutes with continuous or frequent assessment of the response. Intraosseous or sagittal sinus vascular access may be required if venous access is difficult.31 Additional aliquots of fluid should be given under close observation until the patient is normovolemic. Once the patient is normovolemic, inotropic support will be required if the patient still exhibits symptoms of shock. Patients who are hypovolemic from gastroenteritis do not require colloid as volume replacement.
Fluid and Electrolyte Abnormalities
Once intravascular volume has been restored, attention should be paid to the management of the sodium derangements and dehydration (see Chapter 67). Normal hydration should be achieved over 48 to 72 hours. If renal function is normal, the kidneys will resolve most of the electrolyte abnormalities given adequate treatment of shock and gradual replacement of fluid and electrolyte deficits.
Calculation of fluid therapy is more difficult in the presence of ongoing diarrhea, which may amount to 300 mL/kg in 24 hours. Fluid therapy is best monitored using a combination of serial weighing (if accurate scales are available) and electrolyte measurement. If any doubt exists about urine output, a urinary catheter should be inserted to help distinguish between renal losses and diarrhea. A “metabolic bed” may be used so that stool and urine output can be measured separately. Oliguric renal failure is common, but polyuric renal failure also may occur, particularly in children with severe hypokalemia or hyperglycemia.
Sodium Abnormalities
Hypernatremia may result from excessive water loss, excessive salt intake (usually in poorly constituted rehydration solutions), or a combination of both. It is associated with significant morbidity (especially of the central nervous system) and mortality. Hypernatremia is more common in infancy than in later life and may be associated with the use of formula to feed infants.3
Once shock has been treated, the goal of therapy is to reduce the sodium concentration no faster than 0.5 mEq/L/h.32 Once hypovolemia has been corrected, the remaining fluid deficit should be replaced over 48 to 72 hours using a solution containing approximately 70 mEq/L of sodium.
Low Birth Weight
Babies born weighing less than 2500 g are said to have a low birth weight. Approximately 1 million low-birth-weight infants die each year (see Table 16-1 and Chapter 46). Small-for-gestational-age births are relatively more common in developing countries than in developed countries. The decision about whether a low-birth-weight baby should be considered premature, small for gestational age, or both is important. Problems associated with prematurity (i.e., babies younger than 37 weeks of gestation) include respiratory distress syndrome, apnea, poor feeding, intraventricular hemorrhage, jaundice, infection, and hypothermia. Problems associated with infants who have a small-for-gestational-age status are intrauterine hypoxia, birth asphyxia, meconium aspiration, hypoglycemia, and infection.
Only the general principles of management of low-birth-weight infants are outlined in this chapter; more detailed information is available elsewhere.14,33 Vitamin K (1 mg) is administered intramuscularly after birth. The baby should be kept warm (i.e., well wrapped up in a room kept at 27° to 30°C, which is crucially important) and handled as little as possible. Apnea monitors should be used for babies younger than 32 weeks of gestation, and caffeine or aminophylline should be provided if apnea occurs. Cultures are obtained and penicillin and gentamicin administered if there are any signs of infection, including respiratory distress. Use of breast milk and strict handwashing procedures help prevent cross-infection in the nursery. Desaturation can be treated with nasopharyngeal CPAP. Intubation and mechanical ventilation may be helpful if facilities are available, but nasal CPAP is much safer and often is just as effective.34,35
Kangaroo care is defined as skin-to-skin contact between the mother and baby with frequent and exclusive or nearly exclusive breastfeeding and early discharge from hospital. Many of the controlled trials of kangaroo care are of poor quality. A Cochrane Review concluded that kangaroo care appears to reduce morbidity, but more well-designed trials are needed.36
Poliomyelitis, bacille Calmette-Guérin (BCG), and hepatitis B vaccines should be administered before discharge at a time when the baby is fully breast-fed and gaining weight. The mother should be given a solution of ferrous sulfate to give to her child at a dose of 2 mg/kg/day of elemental iron, and follow-up should be scheduled.
Neonatal Asphyxia
Approximately 900,000 infants die every year from asphyxia (Table 16-1).10,37 This condition may be caused by an hypoxic-ischemic insult during labor or by fetal abnormalities that were present before labor. Asphyxia is more common in small-for-gestational-age babies and carries a grave prognosis in preterm babies. Even if improvement occurs during the first few hours after delivery, the baby should be observed closely for at least 24 hours because rapid deterioration may occur at 6 to 24 hours as cerebral edema develops.
The baby’s temperature should be kept below 37° C. Controlled trials suggest that mild hypothermia with a core temperature of 32° to 33° C is beneficial.38–40 The blood glucose should be kept in the normal range.
Close surveillance should be undertaken for neonatal sepsis, which can be manifested as asphyxia. Treatment with penicillin and gentamicin is appropriate, and cefotaxime or ceftriaxone should be added if evidence of meningitis is present. Convulsions should be treated with phenobarbital, 20 mg/kg, administered intravenously over 60 minutes. One small study reported that administration of 40 mg/kg of IV phenobarbital to all babies with asphyxia was beneficial,41 but this treatment may cause dangerous levels of sedation unless ventilation is performed.
Malaria
Severe malaria is caused by Plasmodium falciparum, which is the only species of malaria that causes parasitized erythrocytes to adhere to endothelial cells and produce microvascular disease. At any one time, more than 1 billion people are infected with malaria, and the disease causes 0.9 million deaths each year.10,42
Diagnosis
The diagnosis is made by examination of thick and thin blood smears (false-negative results may occur with inexperienced staff) or by detection of P. falciparum antigen using enzyme-linked immunosorbent assay, polymerase chain reaction (PCR), or immunoassay for parasite lactate dehydrogenase. Commercially available rapid blood tests use a dipstick or test strip with monoclonal antibodies directed against a parasite antigen.42 They have a sensitivity and specificity greater than 90% for P. falciparum.
Initial Treatment
Patients exhibiting coma or shock should be intubated and undergo ventilation. Insertion of a femoral venous cannula to allow monitoring of central venous pressure and infusion of drugs should be considered. Hypovolemia should be corrected with 10 mL/kg boluses of 0.9% saline solution; if it is available, 4% to 5% albumin may be preferable to saline solution.43 If poor perfusion persists despite an adequate central venous pressure, echocardiography may aid in assessing intravascular volume and ventricular contractility (see Chapter 23). Hypoglycemia is common in small children, and their blood glucose concentration should be monitored closely. Convulsions are treated with IV phenobarbital, 20 mg/kg administered over 1 hour, then 5 mg/kg daily.
Routine use of phenobarbital may increase the incidence of mortality in patients who do not undergo ventilation but likely does not do so in patients who do undergo ventilation.44 Administration of pentoxifylline, N-acetylcysteine, and mannitol probably is not helpful, but use of levamisole may be beneficial.45
Antimalarial Drugs
The choice of antimalarial drug depends on which drugs are available and on local drug resistance patterns. The best choice usually is either quinine or artesunate. Evidence suggests that artesunate clears parasites faster than quinine does and increases survival rates,46 but resistance has been reported recently in Southeast Asia.47 Patients with severe malaria are infected by a large number of parasites and should always be treated with two antimalarial drugs to reduce the risk of selecting resistant strains with recrudescence of disease.48
Artesunate is given in an initial IM or IV dose of 2 mg/kg, then 1 mg/kg/day after 6 hours if the infection is hyperparasitic, then 2 mg/kg daily until the child can swallow.49,50 When the child can swallow, artesunate should be administered orally. After 7 days of therapy with artesunate, oral mefloquine, 15 mg/kg followed by 10 mg/kg 12 hours later, should be given.
Quinidine can be used if artesunate and quinine are not available, but quinidine is more likely than quinine to cause cardiac toxicity.15 Following an IV loading dose of 15 mg/kg quinidine over 4 hours, the drug is administered at a dose of 7.5 mg/kg over 4 hours, every 8 hours, for 7 days. After 7 days of therapy with quinidine, mefloquine, 15 mg/kg orally followed by 10 mg/kg 12 hours later, should be given.
Other Treatment
Children with a hemoglobin level of 4 g/dL or less require urgent transfusion with packed erythrocytes. Children with a hemoglobin level of 4 to 6 g/dL should receive a transfusion if they are in a coma or in shock or have more than 10% parasitized erythrocytes.15,49 Transfusion should occur slowly over 6 hours until a hemoglobin level of 10 g/dL is achieved, taking care to avoid fluid overload in children with severe malnutrition. Use of furosemide is not indicated unless there is evidence of fluid overload (i.e., pulmonary edema with a high central venous pressure). Exchange transfusion, if it can be performed safely, may be beneficial in patients who are in a coma or have renal failure, adult respiratory distress syndrome, or a parasitemia of 10% or more.51
No evidence supports the use of corticosteroids,52 cyclosporin, dextran, heparin, iron chelating agents,53 or prostacyclin in patients with malaria. The patient should be turned every 2 hours, hypoglycemia should be prevented, and vital signs and fluid balance should be monitored carefully. Acidosis may be associated with severe hyperkalemia initially, but total body potassium often is low and hypokalemia may occur in the recovery phase.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree