Key Concepts
 Neonates and infants have fewer and smaller alveoli, reducing lung compliance; in contrast, their cartilaginous rib cage makes their chest wall very compliant. The combination of these two characteristics promotes chest wall collapse during inspiration and relatively low residual lung volumes at expiration. The resulting decrease in functional residual capacity (FRC) limits oxygen reserves during periods of apnea (eg, intubation attempts) and readily predisposes them to atelectasis and hypoxemia.
Neonates and infants have fewer and smaller alveoli, reducing lung compliance; in contrast, their cartilaginous rib cage makes their chest wall very compliant. The combination of these two characteristics promotes chest wall collapse during inspiration and relatively low residual lung volumes at expiration. The resulting decrease in functional residual capacity (FRC) limits oxygen reserves during periods of apnea (eg, intubation attempts) and readily predisposes them to atelectasis and hypoxemia.
 Compared with older children and adults, neonates and infants have a proportionately larger head and tongue, narrower nasal passages, an anterior and cephalad larynx, a longer epiglottis, and a shorter trachea and neck. These anatomic features make neonates and infants obligate nasal breathers until about 5 months of age. The cricoid cartilage is the narrowest point of the airway in children younger than 5 years of age.
Compared with older children and adults, neonates and infants have a proportionately larger head and tongue, narrower nasal passages, an anterior and cephalad larynx, a longer epiglottis, and a shorter trachea and neck. These anatomic features make neonates and infants obligate nasal breathers until about 5 months of age. The cricoid cartilage is the narrowest point of the airway in children younger than 5 years of age.
 Cardiac stroke volume is relatively fixed by a noncompliant and immature left ventricle in neonates and infants. The cardiac output is therefore very sensitive to changes in heart rate.
Cardiac stroke volume is relatively fixed by a noncompliant and immature left ventricle in neonates and infants. The cardiac output is therefore very sensitive to changes in heart rate.
 Thin skin, low fat content, and a greater surface area relative to weight promote greater heat loss to the environment in neonates. Heat loss is compounded by cold operating rooms, wound exposure, intravenous fluid administration, dry anesthetic gases, and the direct effect of anesthetic agents on temperature regulation. Hypothermia has been associated with delayed awakening from anesthesia, cardiac irritability, respiratory depression, increased pulmonary vascular resistance, and altered drug responses.
Thin skin, low fat content, and a greater surface area relative to weight promote greater heat loss to the environment in neonates. Heat loss is compounded by cold operating rooms, wound exposure, intravenous fluid administration, dry anesthetic gases, and the direct effect of anesthetic agents on temperature regulation. Hypothermia has been associated with delayed awakening from anesthesia, cardiac irritability, respiratory depression, increased pulmonary vascular resistance, and altered drug responses.
 Neonates, infants, and young children have relatively greater alveolar ventilation and reduced FRC compared with older children and adults even after adjustment for weight. This greater minute ventilation-to-FRC ratio with relatively greater blood flow to vessel-rich organs contributes to a rapid increase in alveolar anesthetic concentration and speeds inhalation induction.
Neonates, infants, and young children have relatively greater alveolar ventilation and reduced FRC compared with older children and adults even after adjustment for weight. This greater minute ventilation-to-FRC ratio with relatively greater blood flow to vessel-rich organs contributes to a rapid increase in alveolar anesthetic concentration and speeds inhalation induction.
 Minimum alveolar concentration (MAC) for halogenated agents is greater in infants than in neonates and adults. Unlike other agents, sevoflurane has the same MAC in neonates and infants. Sevoflurane appears to have a greater therapeutic index than halothane and has become the preferred agent for inhaled induction in pediatric anesthesia.
Minimum alveolar concentration (MAC) for halogenated agents is greater in infants than in neonates and adults. Unlike other agents, sevoflurane has the same MAC in neonates and infants. Sevoflurane appears to have a greater therapeutic index than halothane and has become the preferred agent for inhaled induction in pediatric anesthesia.
 Children are more susceptible than adults to cardiac arrhythmias, hyperkalemia, rhabdomyolysis, myoglobinemia, masseter spasm, and malignant hyperthermia associated with succinylcholine. When a child experiences cardiac arrest following administration of succinylcholine, immediate treatment for hyperkalemia should be instituted.
Children are more susceptible than adults to cardiac arrhythmias, hyperkalemia, rhabdomyolysis, myoglobinemia, masseter spasm, and malignant hyperthermia associated with succinylcholine. When a child experiences cardiac arrest following administration of succinylcholine, immediate treatment for hyperkalemia should be instituted.
 Unlike adults, children may have profound bradycardia and sinus node arrest following the first dose of succinylcholine without atropine pretreatment.
Unlike adults, children may have profound bradycardia and sinus node arrest following the first dose of succinylcholine without atropine pretreatment.
 A viral infection within 2-4 weeks before general anesthesia and endotracheal intubation appears to place the child at an increased risk for perioperative pulmonary complications, such as wheezing, laryngospasm, hypoxemia, and atelectasis.
A viral infection within 2-4 weeks before general anesthesia and endotracheal intubation appears to place the child at an increased risk for perioperative pulmonary complications, such as wheezing, laryngospasm, hypoxemia, and atelectasis.
 Temperature must be closely monitored in pediatric patients because of their greater risk for malignant hyperthermia and the potential for both iatrogenic hypothermia and hyperthermia.
Temperature must be closely monitored in pediatric patients because of their greater risk for malignant hyperthermia and the potential for both iatrogenic hypothermia and hyperthermia.
 Meticulous attention to fluid intake and loss is required in younger pediatric patients because these patients have limited margins of error. A programmable infusion pump or a buret with a microdrip chamber is useful for accurate measurements. Drugs can be flushed through low dead-space tubing to minimize unnecessary fluid administration.
Meticulous attention to fluid intake and loss is required in younger pediatric patients because these patients have limited margins of error. A programmable infusion pump or a buret with a microdrip chamber is useful for accurate measurements. Drugs can be flushed through low dead-space tubing to minimize unnecessary fluid administration.
 Laryngospasm can usually be avoided by extubating the patient either while awake or while deeply anesthetized; both techniques have advocates. Extubation during the interval between these extremes, however, is generally recognized as more hazardous.
Laryngospasm can usually be avoided by extubating the patient either while awake or while deeply anesthetized; both techniques have advocates. Extubation during the interval between these extremes, however, is generally recognized as more hazardous.
 Patients with scoliosis due to muscular dystrophy are predisposed to malignant hypertension, cardiac arrhythmias, and untoward effects of succinylcholine (hyperkalemia, myoglobinuria, and sustained muscular contractures).
Patients with scoliosis due to muscular dystrophy are predisposed to malignant hypertension, cardiac arrhythmias, and untoward effects of succinylcholine (hyperkalemia, myoglobinuria, and sustained muscular contractures).
Pediatric Anesthesia: Introduction
Pediatric anesthesia involves more than simply adjusting drug doses and equipment for smaller patients. Neonates (0-1 months), infants (1-12 months), toddlers (12-24 months), and young children (2-12 years of age) have differing anesthetic requirements. Safe anesthetic management depends on full appreciation of the physiological, anatomic, and pharmacological characteristics of each group (Table 42-1). Indeed infants are at much greater risk of anesthetic morbidity and mortality than older children; risk is generally inversely proportional to age. In addition, pediatric patients are prone to illnesses that require unique surgical and anesthetic strategies.
|
|
|
The transition from fetal to neonatal physiology is reviewed in Chapter 40. Compared with older children and adults, neonates and infants have weaker intercostal muscles and weaker diaphragms (due to a paucity of type I fibers) and less efficient ventilation, more horizontal and pliable ribs, and protuberant abdomens. Respiratory rate is increased in neonates and gradually falls to adult values by adolescence. Tidal volume and dead space per kilogram are nearly constant during development. The presence of fewer, smaller airways produces increased airway resistance. The alveoli are fully mature by late childhood (about 8 years of age). The work of breathing is increased and respiratory muscles easily fatigue.  Neonates and infants have fewer and smaller alveoli, reducing lung compliance; in contrast, their cartilaginous rib cage makes their chest wall very compliant. The combination of these two characteristics promotes chest wall collapse during inspiration and relatively low residual lung volumes at expiration. The resulting decrease in functional residual capacity (FRC) limits oxygen reserves during periods of apnea (eg, intubation attempts) and readily predisposes neonates and infants to atelectasis and hypoxemia. This may be exaggerated by their relatively higher rate of oxygen consumption. Moreover, hypoxic and hypercapnic ventilatory drives are not well developed in neonates and infants. In fact, unlike in adults, hypoxia and hypercapnia may depress respiration in these patients.
Neonates and infants have fewer and smaller alveoli, reducing lung compliance; in contrast, their cartilaginous rib cage makes their chest wall very compliant. The combination of these two characteristics promotes chest wall collapse during inspiration and relatively low residual lung volumes at expiration. The resulting decrease in functional residual capacity (FRC) limits oxygen reserves during periods of apnea (eg, intubation attempts) and readily predisposes neonates and infants to atelectasis and hypoxemia. This may be exaggerated by their relatively higher rate of oxygen consumption. Moreover, hypoxic and hypercapnic ventilatory drives are not well developed in neonates and infants. In fact, unlike in adults, hypoxia and hypercapnia may depress respiration in these patients.
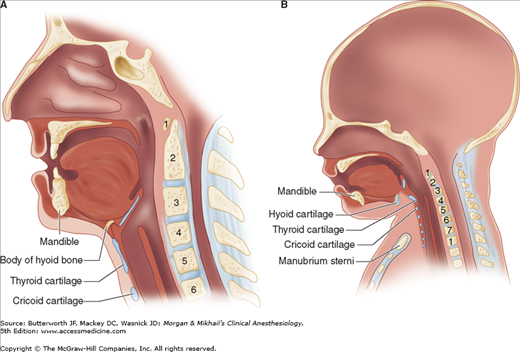
 Neonates and infants have, compared with older children and adults, a proportionately larger head and tongue, narrower nasal passages, an anterior and cephalad larynx (the glottis is at a vertebral level of C4 versus C6 in adults), a longer epiglottis, and a shorter trachea and neck (Figure 42-1). These anatomic features make neonates and young infants obligate nasal breathers until about 5 months of age. The cricoid cartilage is the narrowest point of the airway in children younger than 5 years of age; in adults, the narrowest point is the glottis. One millimeter of mucosal edema will have a proportionately greater effect on gas flow in children because of their smaller tracheal diameters.
Neonates and infants have, compared with older children and adults, a proportionately larger head and tongue, narrower nasal passages, an anterior and cephalad larynx (the glottis is at a vertebral level of C4 versus C6 in adults), a longer epiglottis, and a shorter trachea and neck (Figure 42-1). These anatomic features make neonates and young infants obligate nasal breathers until about 5 months of age. The cricoid cartilage is the narrowest point of the airway in children younger than 5 years of age; in adults, the narrowest point is the glottis. One millimeter of mucosal edema will have a proportionately greater effect on gas flow in children because of their smaller tracheal diameters.
 Cardiac stroke volume is relatively fixed by a noncompliant and immature left ventricle in neonates and infants. The cardiac output is therefore very sensitive to changes in heart rate (see Chapter 20). Although basal heart rate is greater than in adults (Table 42-2), activation of the parasympathetic nervous system, anesthetic overdose, or hypoxia can quickly trigger bradycardia and profound reductions in cardiac output. Sick infants undergoing emergency or prolonged surgical procedures appear particularly prone to episodes of bradycardia that can lead to hypotension, asystole, and intraoperative death. The sympathetic nervous system and baroreceptor reflexes are not fully mature. The infant cardiovascular system displays a blunted response to exogenous catecholamines. The immature heart is more sensitive to depression by volatile anesthetics and to opioid-induced bradycardia. The vascular tree is less able to respond to hypovolemia with compensatory vasoconstriction. Intravascular volume depletion in neonates and infants may be signaled by hypotension without tachycardia.
Cardiac stroke volume is relatively fixed by a noncompliant and immature left ventricle in neonates and infants. The cardiac output is therefore very sensitive to changes in heart rate (see Chapter 20). Although basal heart rate is greater than in adults (Table 42-2), activation of the parasympathetic nervous system, anesthetic overdose, or hypoxia can quickly trigger bradycardia and profound reductions in cardiac output. Sick infants undergoing emergency or prolonged surgical procedures appear particularly prone to episodes of bradycardia that can lead to hypotension, asystole, and intraoperative death. The sympathetic nervous system and baroreceptor reflexes are not fully mature. The infant cardiovascular system displays a blunted response to exogenous catecholamines. The immature heart is more sensitive to depression by volatile anesthetics and to opioid-induced bradycardia. The vascular tree is less able to respond to hypovolemia with compensatory vasoconstriction. Intravascular volume depletion in neonates and infants may be signaled by hypotension without tachycardia.
Pediatric patients have a larger surface area per kilogram than adults (or a smaller body-mass index). Metabolism and its associated parameters (oxygen consumption, CO2 production, cardiac output, and alveolar ventilation) correlate better with surface area than with weight.
 Thin skin, low fat content, and a greater surface area relative to weight promote greater heat loss to the environment in neonates. This problem is compounded by inadequately warmed operating rooms, prolonged wound exposure, administration of room temperature intravenous or irrigation fluid, and dry anesthetic gases. Of course, there are also effects of anesthetic agents on temperature regulation (see Chapter 52). Even mild degrees of hypothermia can cause perioperative problems, including delayed awakening from anesthesia, cardiac irritability, respiratory depression, increased pulmonary vascular resistance, and altered responses to anesthetics, neuromuscular blockers, and other agents. The more important mechanisms for heat production in neonates are nonshivering thermogenesis by metabolism of brown fat and shifting of hepatic oxidative phosphorylation to a more thermogenic pathway. Yet, metabolism of brown fat is severely limited in premature infants and in sick neonates who are deficient in fat stores. Furthermore, volatile anesthetics inhibit thermogenesis in brown adipocytes.
Thin skin, low fat content, and a greater surface area relative to weight promote greater heat loss to the environment in neonates. This problem is compounded by inadequately warmed operating rooms, prolonged wound exposure, administration of room temperature intravenous or irrigation fluid, and dry anesthetic gases. Of course, there are also effects of anesthetic agents on temperature regulation (see Chapter 52). Even mild degrees of hypothermia can cause perioperative problems, including delayed awakening from anesthesia, cardiac irritability, respiratory depression, increased pulmonary vascular resistance, and altered responses to anesthetics, neuromuscular blockers, and other agents. The more important mechanisms for heat production in neonates are nonshivering thermogenesis by metabolism of brown fat and shifting of hepatic oxidative phosphorylation to a more thermogenic pathway. Yet, metabolism of brown fat is severely limited in premature infants and in sick neonates who are deficient in fat stores. Furthermore, volatile anesthetics inhibit thermogenesis in brown adipocytes.
Kidney function approaches normal values (corrected for size) by 6 months of age, but this may be delayed until the child is 2 years old. Premature neonates often demonstrate multiple forms of renal immaturity, including decreased creatinine clearance; impaired sodium retention, impaired glucose excretion, and impaired bicarbonate reabsorption; and reduced diluting and concentrating ability. These abnormalities underscore the importance of appropriate fluid administration in the early days of life.
Neonates also have a relatively increased incidence of gastroesophageal reflux. The immature liver conjugates drugs and other molecules less readily early in life.
Neonates have relatively reduced glycogen stores, predisposing them to hypoglycemia. Impaired glucose excretion by the kidneys may partially offset this tendency. In general, neonates at greatest risk for hypoglycemia are either premature or small for gestational age, receiving hyperalimentation, and the offspring of diabetic mothers.
Pediatric drug dosing is typically adjusted on a per-kilogram basis for convenience (Table 42-3). In early childhood a patient’s weight can be approximated based on age:
50th percentile weight (kg) = (Age × 2) + 9
| Drug | Comment | Dosage |
|---|---|---|
| Acetaminophen | Rectal | 40 mg/kg |
| PO | 10-20 mg/kg | |
| IV (age > 2 y) | 15 mg/kg | |
| Maximum (per day) | 60 mg/kg | |
| Adenosine | Rapid IV bolus | 0.1 mg/kg |
| Repeat dose | 0.2 mg/kg | |
| Maximum dose | 12 mg | |
| Albuterol | Nebulized | 1.25-2.5 mg in 2 mL saline |
| Alfentanil | Anesthetic supplement (IV) | 20-25 mcg/kg |
| Maintenance infusion | 1-3 mcg/kg/min | |
| Aminophylline | Loading dose administered over 20 min (IV) | 5-6 mg/kg |
| Maintenance dose (therapeutic level: 10-20 mg/mL) | 0.5-0.9 mg/kg/h | |
| Amiodarone | Loading dose (IV) | 5 mg/kg |
| Repeat dose (slowly) | 5 mg/kg | |
| Infusion | 5-10 mcg/ kg/min | |
| Maximum dose | 20 mg/kg/day | |
| Amoxicillin | PO | 50 mg/kg |
| Ampicillin | IV | 50 mg/kg |
| Ampicillin/ sulbactam | IV | 25-50 mg/kg |
| Atracurium | Intubation (IV) | 0.5 mg/kg |
| Atropine | IV | 0.01-0.02 mg/kg |
| IM | 0.02 mg/kg | |
| Minimum dose | 0.1 mg | |
| Premedication (PO) | 0.03-0.05 mg/kg | |
| Bretylium | Loading dose (IV) | 5 mg/kg |
| Caffeine | IV | 10 mg/kg |
| Calcium chloride | IV (slowly) | 5-20 mg/kg |
| Calcium gluconate | IV (slowly) | 15-100 mg/kg |
| Cefazolin | IV | 25 mg/kg |
| Cefotaxime | IV | 25-50 mg/kg |
| Cefotetan | IV | 20-40 mg/kg |
| Cefoxitin | IV | 30-40 mg/kg |
| Ceftazidime | IV | 30-50 mg/kg |
| Ceftriaxone | IV | 25-50 mg/kg |
| Cefuroxime | IV | 25 mg/kg |
| Chloral hydrate | PO | 25-100 mg/kg |
| Rectal | 50 mg/kg | |
| Cimetidine | IV or PO | 5-10 mg/kg |
| Cisatracurium | Intubation (IV) | 0.15 mg/kg |
| Clindamycin | IV | 20 mg/kg |
| Dantrolene | Initial dose (IV) | 2.5 mg/kg |
| Maximum dose | 10 mg/kg | |
| Subsequent attempts | 4 J/kg | |
| Desmopressin | IV | 0.2-0.4 mcg/kg |
| Dexamethasone | IV | 0.1-0.5 mg/kg |
| Dextrose | D25W or D50W (IV) | 0.5-1 g/kg |
| Digoxin | IV | 0.1-0.2 mg/kg |
| Three divided doses over 24 h (IV) | 15-30 mcg/kg | |
| Diltiazem | IV over 2 min | 0.25 mg/kg |
| Diphenhydramine | IV, IM, or PO | 1 mg/kg |
| Dobutamine | Infusion | 2-20 mcg/kg/min |
| Dolasetron | IV | 0.35 mg/kg |
| Dopamine | Infusion | 2-20 mcg/kg/min |
| Droperidol | IV | 50-75 mcg/kg |
| Edrophonium | Depends on degree of paralysis (IV) | 0.5-1 mg/kg |
| Ephedrine | IV | 0.1-0.3 mg/kg |
| Epinephrine | IV bolus | 10 mcg/kg |
| Endotracheal dose | 100 mcg/kg | |
| Infusion | 0.05-1 mcg/kg/min | |
| Epinephrine, 2.25% racemic | Nebulized | 0.05 mL/kg in 3 mL saline |
| Esmolol | IV bolus | 100-500 mcg/kg |
| IV infusion | 25-200 mcg/kg/min | |
| Famotidine | IV | 0.15 mg/kg |
| Fentanyl | Pain relief (IV) | 1-2 mcg/kg |
| Pain relief (Intranasal) | 2 mcg/kg | |
| Premedication (Actiq PO) | 10-15 mcg/kg | |
| Anesthetic adjunct (IV) | 1-5 mcg/kg | |
| Maintenance infusion | 2-4 mcg/kg/h | |
| Main anesthetic (IV) | 50-100 mcg/kg | |
| Flumazenil | IV | 0.01 mg/kg |
| Fosphenytoin | IV | 15-20 mg/kg |
| Furosemide | IV | 0.2-1 mg/kg |
| Gentamicin | IV | 2 mg/kg |
| Glucagon | IV | 0.5-1 mg |
| Glucose | IV | 0.5-1 g/kg |
| Glycopyrrolate | IV | 0.01 mg/kg |
| Granisetron | IV | 0.04 mg/kg |
| Heparin | IV (not for cardiac surgery) | 100 units/kg |
| Cardiac surgery dose | 300-400 units/kg | |
| Hydrocortisone | IV | 1 mg/kg |
| Hydromorphone | IV | 15-20 mcg/kg |
| Ibuprofen | PO | 4-10 mg/kg |
| Imipenem | IV | 15-25 mg/kg |
| Inamrinone | Loading (IV) | 1.5 mg/kg |
| Maintenance | 5-10 mcg/kg/min | |
| Insulin | Infusion | 0.02-0.1 units/kg/h |
| Isoproterenol | Infusion | 0.1-1 mcg/kg/min |
| Ketamine | Induction (IV) | 1-2 mg/kg |
| Induction (IM) | 6-10 mg/kg | |
| Induction (per rectum) | 10 mg/kg | |
| Maintenance infusion | 25-75 mcg/kg/min | |
| Premedication (PO) | 6-10 mg/kg | |
| Sedation (IV) | 0.5-1 mg/kg | |
| Ketorolac | IV | 0.5-0.75 mg/kg |
| Labetalol | IV | 0.25 mg/kg |
| Lidocaine | Loading | 1 mg/kg |
| Maintenance | 20-50 mcg/kg/min | |
| Magnesium | IV (slowly) | 25-50 mg/kg |
| sulphate | Maximum single dose | 2 g |
| Mannitol | IV | 0.25-1 g/kg |
| Meperidine | Pain relief (IV) | 0.2-0.5 mg/kg |
| Methohexital | Induction (IV) | 1-2 mg/kg |
| Induction (per rectum) | 25-30 mg/kg | |
| Induction (IM) | 10 mg/kg | |
| Methylprednisolone | IV | 2-4 mg/kg |
| Metoclopramide | IV | 0.15 mg/kg |
| Metronidazole | IV | 7.5 mg/kg |
| Midazolam | Premedication (PO) | 0.5 mg/kg |
| Maximum dose (PO) | 20 mg | |
| Sedation (IM) | 0.1-0.15 mg/kg | |
| Sedation (IV) | 0.05 mg/kg | |
| Milrinone | Loading (IV) | 50-75 mcg/kg |
| Maintenance | 0.375-0.75 mcg/kg/min | |
| Morphine | Pain relief (IV) | 0.025-0.1 mg/kg |
| Premedication (IM) | 0.1 mg/kg | |
| Naloxone | IV | 0.01 mg/kg |
| Neostigmine | Depends on degree of paralysis (IV) | 0.04-0.07 mg/kg |
| Nitroglycerin | IV | 0.5-3 mcg/kg/min |
| Nitroprusside | Infusion | 0.5-4 mcg/kg/min |
| Norepinephrine | Infusion | 0.05-2 mcg/kg/min |
| Ondansetron | IV | 0.1 mg/kg |
| Oxacillin | IV | 50 mg/kg |
| Pancuronium | IV | 0.1 mg/kg |
| Penicillin G | IV | 50,000 units/kg |
| Pentobarbital | Premedication (IM) | 1-2 mg/kg |
| Phenobarbital | Anticonvulsant dose (IV) | 5-20 mg/kg |
| Phentolamine | IV | 30 mcg/kg |
| Phenylephrine | IV | 1-10 mcg/kg |
| Phenytoin | Slowly IV | 5-20 mg/kg |
| Physostigmine | IV | 0.01-0.03 mg/kg |
| Prednisone | PO | 1 mg/kg |
| Procainamide | Loading dose (IV) | 15 mg/kg |
| Propofol | Induction (IV) | 2-3 mg/kg |
| Maintenance infusion | 60-250 mcg/kg/min | |
| Propranolol | IV | 10-25 mcg/kg |
| Prostaglandin E1 | Infusion | 0.05-0.1 mcg/kg/min |
| Protamine | IV | 1 mg/100 units heparin |
| Ranitidine | IV | 0.25-1.0 mg/kg |
| Remifentanil | IV bolus | 0.25-1 mcg/kg |
| IV infusion | 0.05-2 mcg/kg/min | |
| Rocuronium | Intubation (IV) | 0.6-1.2 mg/kg |
| Sodium bicarbonate | IV | 1 mEq/kg |
| Succinylcholine | Intubation (IV) | 1-2mg/kg |
| Intubation (IM) | 4 mg/kg | |
| Sufentanil | Premedication (Intranasal) | 2 mcg/kg |
| Anesthetic adjunct (IV) | 0.5-1 mcg/kg | |
| Maintenance infusion | 0.5-2 mcg/kg/h | |
| Main anesthetic | 10-15 mcg/kg(IV) | |
| Thiopental | Induction (IV) | 5-6 mg/kg |
| Trimethoprim/sulfamethoxazole | IV | 4-5 mg/kg |
| Vancomycin | IV | 20 mg/kg |
| Vecuronium | IV | 0.1 mg/kg |
| Verapamil | IV | 0.1-0.3 mg/kg |
Weight-adjustment of drug dosing is incompletely effective because it does not take into account the disproportionately larger pediatric intravascular and extracellular fluid compartments, the immaturity of hepatic biotransformation pathways, increased organ blood flow, decreased protein for drug binding, or higher metabolic rate.
Neonates and infants have a proportionately greater total water content (70-75%) than adults (50-60%). Total body water content decreases while fat and muscle content increase with age. As a direct result, the volume of distribution for most intravenous drugs is disproportionately greater in neonates, infants, and young children, and the optimal dose (per kilogram) is usually greater than in older children and adults. A disproportionately smaller muscle mass in neonates prolongs the clinical duration of action (by delaying redistribution to muscle) of drugs such as thiopental and fentanyl. Neonates also have a relatively decreased glomerular filtration rate, hepatic blood flow, and renal tubular function, and immature hepatic enzyme systems. Increased intraabdominal pressure and abdominal surgery further reduce hepatic blood flow. All these factors may impair renal drug handling, hepatic metabolism, or biliary excretion of drugs in neonates and young infants. Neonates also have decreased protein binding for some drugs, most notably thiopental, bupivacaine, and many antibiotics. In the case of thiopental, increased free drug enhances potency and reduces the induction dose in neonates compared with older children. An increase in free bupivacaine might increase the risk of systemic toxicity.
 Neonates, infants, and young children have relatively greater alveolar ventilation and reduced FRC compared with older children and adults. This greater minute ventilation-to-FRC ratio with relatively greater blood flow to vessel-rich organs contributes to a rapid increase in alveolar anesthetic concentration and speeds inhalation induction. Furthermore, the blood/gas coefficients of volatile anesthetics are reduced in neonates compared with adults, resulting in even faster induction times and potentially increasing the risk of accidental overdosage.
Neonates, infants, and young children have relatively greater alveolar ventilation and reduced FRC compared with older children and adults. This greater minute ventilation-to-FRC ratio with relatively greater blood flow to vessel-rich organs contributes to a rapid increase in alveolar anesthetic concentration and speeds inhalation induction. Furthermore, the blood/gas coefficients of volatile anesthetics are reduced in neonates compared with adults, resulting in even faster induction times and potentially increasing the risk of accidental overdosage.
 The minimum alveolar concentration (MAC) for halogenated agents is greater in infants than in neonates and adults (Table 42-4). In contrast to other agents, no increase in sevoflurane MAC can be demonstrated in neonates and infants. Nitrous oxide does not appear to reduce the MAC of desflurane or sevoflurane in children to the same extent as it does for other agents.
The minimum alveolar concentration (MAC) for halogenated agents is greater in infants than in neonates and adults (Table 42-4). In contrast to other agents, no increase in sevoflurane MAC can be demonstrated in neonates and infants. Nitrous oxide does not appear to reduce the MAC of desflurane or sevoflurane in children to the same extent as it does for other agents.
| Agent | Neonates | Infants | Small Children | Adults |
|---|---|---|---|---|
| Halothane | 0.90 | 1.1-1.2 | 0.9 | 0.75 |
| Sevoflurane | 3.2 | 3.2 | 2.5 | 2 |
| Isoflurane | 1.6 | 1.8-1.9 | 1.3-1.6 | 1.2 |
| Desflurane | 8-9 | 9-10 | 7-8 | 6 |
The blood pressure of neonates and infants appears to be especially sensitive to volatile anesthetics. This clinical observation has been attributed to less-well-developed compensatory mechanisms (eg, vasoconstriction, tachycardia) and greater sensitivity of the immature myocardium to myocardial depressants. Halothane (now much less commonly used) sensitizes the heart to catecholamines. The maximum recommended dose of epinephrine in local anesthetic solutions during halothane anesthesia is 10 mcg/kg. Cardiovascular depression, bradycardia, and arrhythmias are less frequent with sevoflurane than with halothane. Halothane and sevoflurane are less likely than other volatile agents to irritate the airway or cause breath holding or laryngospasm during induction (see Chapter 8). In general, volatile anesthetics appear to depress ventilation more in infants than in older children. Sevoflurane appears to produce the least respiratory depression. The risk for halothane-induced hepatic dysfunction appears to be much reduced in prepubertal children compared with adults. There are no reported instances of renal toxicity attributed to inorganic fluoride production during sevoflurane anesthesia in children. Overall, sevoflurane appears to have a greater therapeutic index than halothane and has become the preferred agent for inhaled induction in pediatric anesthesia.
Emergence is fastest following desflurane or sevoflurane, but both agents are associated with a greater incidence of agitation or delirium upon emergence, particularly in young children. Because of the latter, some clinicians switch to isoflurane for maintenance anesthesia following a sevoflurane induction (see below).
After weight-adjustment of dosing, infants and young children require larger doses of propofol because of a larger volume of distribution compared with adults. Children also have a shorter elimination half-life and higher plasma clearance for propofol. Recovery from a single bolus is not appreciably different from that in adults; however, recovery following a continuous infusion may be more rapid. For the same reasons, children may require increased weight-adjusted rates of infusion for maintenance of anesthesia (up to 250 mcg/kg/min). Propofol is not recommended for prolonged sedation of critically ill pediatric patients in the intensive care unit (ICU) due to an association with greater mortality than other agents. Although the “propofol infusion syndrome” has been reported more often in critically ill children, it has also been reported in adults undergoing long-term propofol infusion (>48 h) for sedation, particularly at increased doses (>5 mg/kg/h). Its essential features include rhabdomyolysis, metabolic acidosis, hemodynamic instability, hepatomegaly, and multiorgan failure.
Children require relatively larger doses of thiopental compared with adults. The elimination half-life is shorter and the plasma clearance is greater than in adults. In contrast, neonates, appear to be more sensitive to barbiturates. Neonates have less protein binding, a longer half-life, and impaired clearance. The thiopental induction dose for neonates is 3-4 mg/kg compared with 5-6 mg/kg for infants.
Opioids appear to be more potent in neonates than in older children and adults. Unproven (but popular) explanations include “easier entry” across the blood-brain barrier, decreased metabolic capability, or increased sensitivity of the respiratory centers. Morphine sulfate, particularly in repeated doses, should be used with caution in neonates because hepatic conjugation is reduced and renal clearance of morphine metabolites is decreased. The cytochrome P-450 pathways mature at the end of the neonatal period. Older pediatric patients have relatively greater rates of biotransformation and elimination as a result of high hepatic blood flow. Sufentanil, alfentanil, and, possibly, fentanyl clearances may be greater in children than in adults. Remifentanil clearance is increased in neonates and infants but elimination half-life is unaltered compared with adults. Neonates and infants may be more resistant to the hypnotic effects of ketamine, requiring slightly higher doses than adults (but the “differences” are within the range of error in studies); pharmacokinetic values do not appear to be significantly different from those of adults. Etomidate has not been well-studied in pediatric patients younger than 10 years of age; its profile in older children is similar to that in adults. Midazolam has the fastest clearance of all the benzodiazepines; however, midazolam clearance is significantly reduced in neonates compared with older children. The combination of midazolam and fentanyl can cause hypotension in patients of all ages.
For a wide variety of reasons (including pharmacology, case mix, and convenience), muscle relaxants are less commonly used during induction of anesthesia in pediatric than in adult patients. Many children will have a laryngeal mask airway (LMA) or endotracheal tube placed after receiving a sevoflurane inhalation induction, placement of an intravenous catheter, and administration of various combinations of propofol, opioids, or lidocaine.
All muscle relaxants generally have a faster onset (up to 50% less delay) in pediatric patients because of shorter circulation times than adults. In both children and adults, intravenous succinylcholine (1-1.5 mg/kg) has the fastest onset (see Chapter 11). Infants require significantly larger doses of succinylcholine (2-3 mg/kg) than older children and adults because of the relatively larger volume of distribution. This discrepancy disappears if dosage is based on body surface area. Table 42-5 lists commonly used muscle relaxants and their ED95 (the dose that produces 95% depression of evoked twitches). With the notable exclusion of succinylcholine and possibly cisatracurium, infants require significantly smaller muscle relaxant doses than older children. Moreover, based on weight, older children require larger doses than adults for some neuromuscular blocking agents (eg, atracurium, see Chapter 11). As with adults, a more rapid intubation can be achieved with a muscle relaxant dose that is twice the ED95 dose at the expense of prolonging the duration of action.
| Agents | Infants ED95 (mg/kg) | Children ED95 (mg/kg) |
|---|---|---|
| Succinylcholine | 0.7 | 0.4 |
| Atracurium | 0.25 | 0.35 |
| Cisatracurium | 0.05 | 0.06 |
| Rocuronium | 0.25 | 0.4 |
| Vecuronium | 0.05 | 0.08 |
| Pancuronium | 0.07 | 0.09 |
The response of neonates to nondepolarizing muscle relaxants is variable. Popular (and unproven) explanations for this include “immaturity of the neuromuscular junction” (in premature neonates), tending to increase sensitivity (unproven), counterbalanced by a disproportionately larger extracellular compartment, reducing drug concentrations (proven). The relative immaturity of neonatal hepatic function prolongs the duration of action for drugs that depend primarily on hepatic metabolism (eg, pancuronium, vecuronium, and rocuronium). Atracurium and cisatracurium do not depend on hepatic biotransformation and reliably behave as intermediate-acting muscle relaxants.
 Children are more susceptible than adults to cardiac arrhythmias, hyperkalemia, rhabdomyolysis, myoglobinemia, masseter spasm, and malignant hyperthermia (see Chapter 52) associated with succinylcholine. When a child experiences cardiac arrest following administration of succinylcholine, immediate treatment for hyperkalemia should be instituted. Prolonged, heroic (eg, potentially including cardiopulmonary bypass) resuscitative efforts may be required. For this reason, succinylcholine is avoided for routine, elective paralysis for intubation in children and adolescents.
Children are more susceptible than adults to cardiac arrhythmias, hyperkalemia, rhabdomyolysis, myoglobinemia, masseter spasm, and malignant hyperthermia (see Chapter 52) associated with succinylcholine. When a child experiences cardiac arrest following administration of succinylcholine, immediate treatment for hyperkalemia should be instituted. Prolonged, heroic (eg, potentially including cardiopulmonary bypass) resuscitative efforts may be required. For this reason, succinylcholine is avoided for routine, elective paralysis for intubation in children and adolescents.  Unlike adults, children may have profound bradycardia and sinus node arrest following the first dose of succinylcholine without atropine pretreatment. Atropine (0.1 mg minimum) must therefore always be administered prior to succinylcholine in children. Generally accepted indications for intravenous succinylcholine in children include rapid sequence induction with a “full” stomach and laryngospasm that does not respond to positive-pressure ventilation. When rapid muscle relaxation is required prior to intravenous access (eg, with inhaled inductions in patients with full stomachs), intramuscular succinylcholine (4-6 mg/kg) can be used. Intramuscular atropine (0.02 mg/kg) should be administered with intramuscular succinylcholine to reduce the likelihood of bradycardia. Some clinicians advocate intralingual administration (2 mg/kg in the midline to avoid hematoma formation) as an alternate emergency route for intramuscular succinylcholine.
Unlike adults, children may have profound bradycardia and sinus node arrest following the first dose of succinylcholine without atropine pretreatment. Atropine (0.1 mg minimum) must therefore always be administered prior to succinylcholine in children. Generally accepted indications for intravenous succinylcholine in children include rapid sequence induction with a “full” stomach and laryngospasm that does not respond to positive-pressure ventilation. When rapid muscle relaxation is required prior to intravenous access (eg, with inhaled inductions in patients with full stomachs), intramuscular succinylcholine (4-6 mg/kg) can be used. Intramuscular atropine (0.02 mg/kg) should be administered with intramuscular succinylcholine to reduce the likelihood of bradycardia. Some clinicians advocate intralingual administration (2 mg/kg in the midline to avoid hematoma formation) as an alternate emergency route for intramuscular succinylcholine.
Many clinicians consider rocuronium (0.6 mg/kg intravenously) to be the drug of choice (when a relaxant will be used) during routine intubation in pediatric patients with intravenous access because it has the fastest onset of nondepolarizing neuromuscular blocking agents (see Chapter 11). Larger doses of rocuronium (0.9-1.2 mg/kg) may be used for rapid sequence induction but a prolonged duration (up to 90 min) will likely follow. Rocuronium is the only nondepolarizing neuromuscular blocker that has been adequately studied for intramuscular administration (1.0-1.5 mg/kg), but this approach requires 3-4 min for onset.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree