Opioids
There has been an exponential increase in the prescription of high-dose opioids for the treatment of patients with chronic noncancer pain (3% of the adult US population) (Dahan A, Niesters M, Olofsen E, Smith T, Overdyk F. Opioids. In: Barash PG, Cullen BF, Stoelting RK, Cahalan MK, Ortega R, Stock MC, eds. Clinical Anesthesia. Philadelphia: Lippincott Williams & Wilkins; 2013:501–522). This “epidemic” of opioid use, coupled with an emphasis on aggressive and effective postoperative pain management for patients undergoing surgery, has resulted in increasingly complex postoperative pain management problems for surgical patients and an increase in opioid-related complications for patients with pain patients in general. Expertise in the use of opioids is not only required in the operating room and postoperatively but also when caring for patients with chronic pain in nonsurgical settings.
I. Short History
Opium is among the oldest drugs in the world. (Fossilized opium poppies have been found in Neanderthal excavation sites dating back to 30,000 bce).
The first written reference to the medicinal use of the opium poppy is described in a Sumerian text dated near 4,000 bce.
Just over 200 years ago, the German pharmacist and chemist Friedrich Sertürner isolated a stable alkaloid crystal from the opium sap and named it “morphine” after the Greek god of dreams, Morpheus.
Morphine was tenfold more potent than opium and soon replaced it, not only for the treatment of severe pain but also for a myriad of other purposes such as cough and diarrhea.
After the invention of the hypodermic syringe in the 1850s, the Englishman Alexander Wood was the first to inject morphine in a controlled fashion.
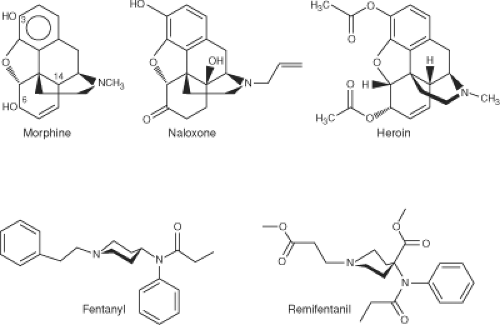
The synthesis of heroin in 1874 was based on the empirical finding that boiling morphine with specific acids caused the replacement of the two morphine —OH groups by —OCOH3 producing diamorphine or heroin (Fig. 19-1).
In 1937, meperidine (pethidine) became the first synthetic opioid synthesized based on the central structure of morphine. Since then, many synthetic and semisynthetic opioids have been produced, including the clinically important opioid antagonists naloxone and naltrexone, by replacing the N-methyl substituent in morphine with allyl and cyclopropylmethyl groups, respectively (see Fig. 19-1).
For clinical use during anesthesia, the most important opioids are the piperidines fentanyl, sufentanil, alfentanil, and remifentanil. These opioids produce potent analgesia and suppression of cardiovascular responses to noxious stimulation from surgery with predictable pharmacokinetics and pharmacodynamics.
The continued development of opioids with complex simultaneous actions at opioid and nonopioid target sites is facilitated by new information gained about mechanisms involved in endogenous pain and analgesia as well as advances in pain-related pharmacology.
Development of new opioids is also driven by concerns that the side effect profile of potent opioids, which presents a serious risk to patients, needs to be minimized.
II. The Endogenous Opiod System
A major breakthrough in the understanding of opioid pharmacology came from a series of discoveries of opioid receptors, endogenous opioid peptides, their encoding genes, and endogenous opioid alkaloids.
The endogenous opioid system is composed of a family of structurally related endogenous peptides that act at a four-member opioid receptor family consisting of the μ-opioid receptor (MOR), κ-opioid receptor (KOR), δ-opioid receptor (DOR), and orphanin FQ/nociception (NOP) receptor.
At least three MOR subtypes have been described: μ1 is predominantly involved in opioid analgesia, μ2 is involved in opioid-induced respiratory depression, and μ3 is involved in opioid-induced immune suppression.
Functional validation of most opioid receptor subtypes awaits the development of antagonists with sufficient selectivity to allow a clear differentiation by effect.
The endogenous opioid peptides include endorphins, enkephalins, and dynorphins, each of which display different affinities for the μ-, κ-, and δ-opioid receptors. β-endorphins have a high affinity for the μ-opioid receptor, met- and leu-enkephalins for the κ-opioid receptor, and dynorphin A for the δ-opioid receptor.
Opioids act not only through central and peripheral neuronal pathways but also via nonneuronal mechanisms, such as actions on the immune system. Opioid receptors are linked to G proteins in the cell membrane and hence are members of the large G-protein–coupled receptor (GPCR) family. GPCRs mediate a cascade of downstream signaling pathways.
III. Opioid Receptor Knockout Mice
A variety of mice lacking various opioid receptors (“knockout mice”) have been bred to understand the molecular targets of exogenous opioids.
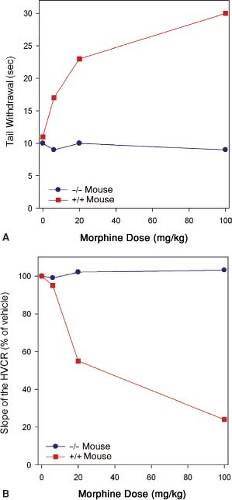
Mice lacking the MOR gene do not experience morphine-induced analgesia, respiratory depression, reward and withdrawal, inhibition of gastrointestinal transit, immunosuppression, or an increase in steroid hormones (Fig. 19-2).
These observations suggest the MOR is the target for both the desired and undesired effects of opioid analgesics. Consequently, designing a MOR-activating drug that selectively produces desired effects such as analgesia, but not undesired effects such as life-threatening respiratory depression, is not possible.
Table 19-1 Classification of Exogenous Opioids | |||||
|---|---|---|---|---|---|
|
IV. Classification of Exogneous Opioids
Opioids may be classified based on their synthesis, chemical structure, potency, receptor binding, and effect at the opioid receptors (Table 19-1). The term opioids is used for all opioids (endogenous opioid peptides, natural opioid alkaloids, semisynthetic opioids, and synthetic opioids).
V. Opioids Acting at Opioid and Nonopioid Receptors
Most opioid analgesics act at multiple receptor systems with different affinities. (Morphine acts with high affinity at MOR and with lower affinities at KOR and DOR.)
Methadone is the most potent NMDA receptor antagonist. Antagonism of the NMDA receptor is clinically useful in
reducing opioid tolerance and opioid-induced hyperalgesia (OIH) and in chronic pain states leading to pain hypersensitivity.
Nonopioids may also act at opioid receptors.
Ketamine is an NMDA receptor antagonist with affinity for multiple receptor systems, including the opioid receptors.
Its anesthetic properties are related to its effect at the NMDA receptors, and its analgesic effects are predominantly caused by MOR activation.
VI. Opioid Mechanisms
Mechanism of Opioid Analgesia
Opioids modify both nociception (reception of signals in the CNS) and the perception of a noxious stimulus (emotional coloring of pain).
Different types of peripheral sensory nociceptors, often free nerve endings, are stimulated by tissue damage, and the resulting pain information is transmitted to the spinal cord by two types of small-diameter peripheral afferent fibers: slow conducting, unmyelinated C fibers (which cause a dull burning pain) and faster, thinly myelinated Aδ fibers (which cause sharp, pricking pain).
μ-Opioid–induced analgesia and descending inhibitory pathways may be activated not only by exogenous opioids but also by activation of endogenous opioid systems.
Stress-induced analgesia. The endogenous opioid system is activated under stressful conditions, as demonstrated by the delayed onset of pain by soldiers wounded in battle.
Placebo-induced analgesia. The endogenous opioid system also mediates placebo-induced analgesia, a reduction of pain resulting from an expectation of pain relief.
Conditioning pain modulation (CPM) is a condition in which pain arising from a noxious stimulus applied to one part of the body is decreased by application of a second remote noxious stimulus. CPM is caused by the activation of descending inhibitory pathways.
Peripheral Opioid Analgesia
Opioids are involved in peripheral analgesia by acting directly on sensory neurons (Aδ and C fibers) to inhibit pain signal transmission (important in inflammatory pain).
The immune system is also widely involved in peripheral analgesia (opioid receptors are located not only on neurons but also on immune cells, such as human leukocytes (Fig. 19-3).
Opioid-Induced Hyperalgesia and Tolerance
Opioids can induce the paradoxical effect of OIH (increase in pain sensitivity), which may limit the analgesic effects of opioids.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree