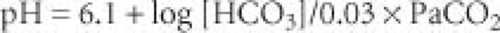Fluids, Electrolytes, and Acid–Base Physiology
Fluids, Electrolytes, and Acid–Base Physiology
As a consequence of underlying diseases and therapeutic manipulations, surgical patients may develop potentially harmful disorders of acid–base equilibrium, intravascular and extravascular volume, and serum electrolytes (Prough DS, Funston JS, Svensen CH, Wolf SW. Fluids, electrolytes, and acid-base physiology. In: Barash PG, Cullen BF, Stoelting RK, Cahalan MK, Ortega R, Stock MC, eds. Clinical Anesthesia. Philadelphia: Lippincott Williams & Wilkins; 2013: 327–361). Precise management of acid–base status, fluids, and electrolytes may limit perioperative morbidity and mortality.
I. Acid–Base Interpretation and Treatment
Management of acid–base disturbances requires an understanding of the four simple acid–base disorders (metabolic alkalosis, metabolic acidosis, respiratory alkalosis, and respiratory acidosis) as well as combinations of more complex disturbances.
Overview of Acid–Base Equilibrium. Conventionally, acid–base equilibrium is described using the Henderson-Hasselbalch equation (
Fig. 14-1). Because the concentration of bicarbonate is largely regulated by the kidneys and CO
2 is controlled by the lungs, acid–base interpretation has emphasized examining disorders in terms of metabolic disturbances (bicarbonate primarily increased or decreased) and respiratory disturbances (PaCO
2 primarily increased or decreased).
The negative logarithm of the hydrogen ion concentration is described as the pH. A pH of 7.4 corresponds to a hydrogen ion concentration of 40 nmol/L.
From a pH of 7.2 to 7.5, the curve of hydrogen ion concentration is relatively linear, and for each change of 0.01 pH unit from 7.4, the hydrogen ion concentration can be estimated to increase (pH >7.4) or decrease (pH >7.4) by 1 nmol/L.
Metabolic alkalosis (pH >7.45 and bicarbonate >27 mEq/L) is the most common acid–base abnormality in critically ill patients (
Tables 14-1 and
14-2).
Metabolic Acidosis (pH <7.35 and bicarbonate <21 mEq/L)
Two types of metabolic acidosis occur based on whether the calculated anion gap is normal or increased (
Table 14-5). The commonly measured cation (sodium) usually exceeds the total concentration of anions (chloride, bicarbonate) by 9 to 13 mEq/L.
Metabolic acidosis exerts multiple physiologic effects (
Table 14-6).
Anesthetic implications of metabolic acidosis are proportional to the severity of the underlying process (
Table 14-7).
Treatment of metabolic acidosis consists of the treatment of the primary pathophysiologic process (hypoperfusion, arterial hypoxemia) and, if pH is severely depressed, administration of sodium bicarbonate (
Table 14-8). Current opinion is that sodium bicarbonate should rarely be used to treat acidemia induced by metabolic acidosis because it does not improve the cardiovascular response to catecholamines and does decrease plasma-ionized calcium.
Respiratory alkalosis (pH > 7.45 and PaCO2 < 35 mm Hg) results from an increase in minute ventilation that is greater than that required to excrete metabolic CO2 production.
The development of spontaneous respiratory alkalosis in a previously normocarbic patient requires prompt evaluation (
Table 14-9).
Respiratory alkalosis exerts multiple physiologic effects (
Table 14-10).
Treatment of respiratory alkalosis per se is often not required. The most important steps are recognition and treatment of the underlying cause (e.g., arterial hypoxemia, hypoperfusion-induced lactic acidosis).
Preoperative recognition of chronic hyperventilation necessitates intraoperative maintenance of a similar PaCO2.
Respiratory acidosis (pH, 7.35; PaCO2 > 45 mm Hg) occurs because of a decrease in minute ventilation and or an increase in production of metabolic CO2.
Respiratory acidosis may be acute (absence of renal bicarbonate retention) or chronic (renal retention of bicarbonate returns the pH to near normal).
Respiratory acidosis occurs because of a decrease in minute ventilation or an increase in CO
2 production (
Table 14-11).
Patients with chronic hypercarbia caused by intrinsic pulmonary disease require careful preoperative evaluation (ABG and pH determinations), anesthetic management (direct arterial blood pressure monitoring and frequent ABG measurements), and postoperative care (pain control, often with neuraxial opioids, and mechanical support of ventilation).
Administration of opioids and sedatives, even in low doses, may cause hazardous depression of ventilation.
Intraoperatively, a patient with chronic hypercapnia should be ventilated to maintain a normal pH. (An abrupt increase in alveolar ventilation may produce profound alkalemia because renal excretion of bicarbonate is slow.)
Treatment of acute respiratory acidosis is elimination of the causative factor (opioids, muscle relaxants) and mechanical support of ventilation as needed. Chronic respiratory acidosis is rarely managed with mechanical ventilation but rather with efforts to improve pulmonary function to permit more effective elimination of CO2.
In patients requiring mechanical ventilation for respiratory failure, ventilation with a lung-protective strategy may result in hypercapnia, which in turn can be managed with alkalinization.
pH = 6.1 + log [HCO3]/0.03 × PaCO2
II. Practical Approach to Acid–Base Interpretation
Rapid interpretation of a patient’s acid–base status involves integration of data provided by ABG, pH, and electrolyte measurements and history. After obtaining these data, a stepwise approach facilitates interpretation (
Table 14-12).
The pH status usually indicates the primary process (acidosis or alkalosis).
If the PaCO
2 and the pH change reciprocally but the magnitude of the pH and bicarbonate changes is not consistent
with a simple acute respiratory disturbance, a chronic respiratory or metabolic problem (>24 hours) should be considered. (pH becomes nearly normal as the body compensates.)
If neither an acute nor a chronic respiratory change could have resulted in the ABG measurements, then a metabolic disturbance must be present.
Compensation in response to metabolic disturbances is prompt via changes in PaCO2, but renal compensation for respiratory disturbances is slower.
Failure to consider the presence or absence of an increased anion gap results in an erroneous diagnosis and failure to initiate appropriate treatment. Correct assessment of the anion gap requires correction for hypoalbuminemia.