Key Concepts
 The most common morbidities encountered in obstetrics are severe hemorrhage and severe preeclampsia.
The most common morbidities encountered in obstetrics are severe hemorrhage and severe preeclampsia.
 Regardless of the time of last oral intake, all obstetric patients are considered to have a full stomach and to be at risk for pulmonary aspiration.
Regardless of the time of last oral intake, all obstetric patients are considered to have a full stomach and to be at risk for pulmonary aspiration.
 Nearly all parenteral opioid analgesics and sedatives readily cross the placenta and can affect the fetus. Regional anesthetic techniques are preferred for management of labor pain.
Nearly all parenteral opioid analgesics and sedatives readily cross the placenta and can affect the fetus. Regional anesthetic techniques are preferred for management of labor pain.
 Using a local anesthetic-opioid mixture for lumbar epidural analgesia during labor significantly reduces drug requirements, compared with using either agent alone.
Using a local anesthetic-opioid mixture for lumbar epidural analgesia during labor significantly reduces drug requirements, compared with using either agent alone.
 Pain relief during labor requires neural blockade at the T10-L1 sensory level in the first stage of labor and at T10-S4 in the second stage.
Pain relief during labor requires neural blockade at the T10-L1 sensory level in the first stage of labor and at T10-S4 in the second stage.
 Continuous lumbar epidural analgesia is the most versatile and most commonly employed technique, because it can be used for pain relief for the first stage of labor as well as analgesia/anesthesia for subsequent vaginal delivery or cesarean section, if necessary.
Continuous lumbar epidural analgesia is the most versatile and most commonly employed technique, because it can be used for pain relief for the first stage of labor as well as analgesia/anesthesia for subsequent vaginal delivery or cesarean section, if necessary.
 When dilute mixtures of a local anesthetic and an opioid are used, epidural analgesia has little if any effect on the progress of labor.
When dilute mixtures of a local anesthetic and an opioid are used, epidural analgesia has little if any effect on the progress of labor.
 Even when aspiration does not yield blood or cerebrospinal fluid, unintentional intravascular or intrathecal placement of an epidural needle or catheter is possible.
Even when aspiration does not yield blood or cerebrospinal fluid, unintentional intravascular or intrathecal placement of an epidural needle or catheter is possible.
 Hypotension is a common side effect of regional anesthetic techniques and must be treated aggressively with phenylephrine or ephedrine, supplemental oxygen, left uterine displacement, and intravenous fluid boluses to prevent fetal compromise.
Hypotension is a common side effect of regional anesthetic techniques and must be treated aggressively with phenylephrine or ephedrine, supplemental oxygen, left uterine displacement, and intravenous fluid boluses to prevent fetal compromise.
 Techniques using combined spinal-epidural analgesia and anesthesia may particularly benefit patients with severe pain early in labor and those who receive analgesia/anesthesia just prior to delivery.
Techniques using combined spinal-epidural analgesia and anesthesia may particularly benefit patients with severe pain early in labor and those who receive analgesia/anesthesia just prior to delivery.
 Spinal or epidural anesthesia is preferred to general anesthesia for cesarean section because regional anesthesia is associated with lower maternal mortality.
Spinal or epidural anesthesia is preferred to general anesthesia for cesarean section because regional anesthesia is associated with lower maternal mortality.
 Continuous epidural anesthesia allows better control over the sensory level than “single-shot” techniques. Conversely, spinal anesthesia has a more rapid, predictable onset; may produce a more dense (complete) block; and lacks the potential for serious systemic drug toxicity because of the smaller dose of local anesthetic employed.
Continuous epidural anesthesia allows better control over the sensory level than “single-shot” techniques. Conversely, spinal anesthesia has a more rapid, predictable onset; may produce a more dense (complete) block; and lacks the potential for serious systemic drug toxicity because of the smaller dose of local anesthetic employed.
 Risk of systemic local anesthetic toxicity during epidural analgesia and anesthesia is minimized by slowly administering dilute solutions for labor pain and by fractionating the total dose administered for cesarean section into 5-mL increments.
Risk of systemic local anesthetic toxicity during epidural analgesia and anesthesia is minimized by slowly administering dilute solutions for labor pain and by fractionating the total dose administered for cesarean section into 5-mL increments.
 Maternal hemorrhage is one of the most common severe morbidities complicating obstetric anesthesia. Causes include placenta previa, abruptio placentae, and uterine rupture.
Maternal hemorrhage is one of the most common severe morbidities complicating obstetric anesthesia. Causes include placenta previa, abruptio placentae, and uterine rupture.
 Common causes of postpartum hemorrhage include uterine atony, a retained placenta, obstetric lacerations, uterine inversion, and use of tocolytic agents prior to delivery.
Common causes of postpartum hemorrhage include uterine atony, a retained placenta, obstetric lacerations, uterine inversion, and use of tocolytic agents prior to delivery.
 Intrauterine asphyxia during labor is the most common cause of neonatal depression. Fetal monitoring throughout labor is helpful in identifying which babies may be at risk, detecting fetal distress, and evaluating the effect of acute interventions.
Intrauterine asphyxia during labor is the most common cause of neonatal depression. Fetal monitoring throughout labor is helpful in identifying which babies may be at risk, detecting fetal distress, and evaluating the effect of acute interventions.
Obstetric Anesthesia: Introduction
This chapter focuses on the practice of obstetric anesthesia. Techniques for analgesia and anesthesia during labor, vaginal delivery, and cesarean section are presented. The chapter ends with a review of neonatal resuscitation.
Although the majority of women of childbearing age are healthy and would be considered to be at minimal operative risk, pregnancy, certain maternal-fetal factors, and preexisting medical conditions significantly increase surgical and obstetric risks.
Maternal mortality is usually presented as the number of women who die while pregnant (or within 42 days of pregnancy termination) after excluding accidents and unrelated causes. This number is often indexed to the total number of live births. The maternal mortality index has decreased nearly 100-fold since 1900. Likely due to better reporting, it rose slightly in the United States to 21 deaths per 100,000 live births in 2010. The world average is 400 deaths per 100,000 live births. Of all maternal deaths worldwide, 99% occur in Africa, Asia, Latin America, and the Caribbean.
In the United States, overall mortality risk is greater for women older than 35 years of age, black women, and women who do not receive prenatal care. The leading causes of death associated with a live birth in 2010 were cardiovascular diseases (13.5%), cardiomyopathy (12.6%), hemorrhage (11.9%), noncardiovascular diseases (11.8%), hypertensive disorders of pregnancy (11.1%), infection/sepsis (11.1%), thrombotic pulmonary embolism (5.6%), amniotic fluid embolism (5.6%), cerebrovascular accidents (5.3%) and anesthesia complications (0.6%) Of all maternal deaths, only 34% of patients died within 24 h of delivery, whereas 55% died between 1 and 42 days, and another 11% died between 43 days and 1 year. Direct causes of maternal deaths are more clearly detailed from Canadian data, which show that, in addition to pulmonary embolism and preeclampsia/pregnancy-induced hypertension (PIH), amniotic fluid embolism and intracranial hemorrhage emerge as important additional causes of death.
Severe obstetric morbidity may be a more sensitive measure of outcome than maternal mortality. Data from the United Kingdom suggest that incidence of severe obstetric morbidity is 12 per 1000 deliveries, 100 times more common than mortality. Risk factors include age greater than 34 years, nonwhite ethnic group, multiple pregnancy, history of hypertension, previous postpartum hemorrhage, and emergency cesarean delivery. Table 41-1 lists the estimated incidence of the most common causes of severe morbidity; thromboembolic disease was deliberately excluded because of the difficulty in making the diagnosis in nonfatal cases.  By far the most common morbidities encountered in obstetrics are severe hemorrhage and severe preeclampsia.
By far the most common morbidities encountered in obstetrics are severe hemorrhage and severe preeclampsia.
Anesthesia accidents and mishaps account for approximately 2-3% of maternal deaths. Data collected between 1985 and 1990 suggested a maternal mortality of 32 deaths per 1,000,000 live births due to general anesthesia and 1.9 deaths per 1,000,000 live births due to regional anesthesia. More recent data between 1998 and 2005 suggest a lower overall maternal mortality from anesthesia (about 1.2 % of live births), possibly due to greater use of regional anesthesia for labor and cesarean delivery. Most deaths occur during or after cesarean section. Moreover, the risk of an adverse outcome appears to be much greater with emergent than with elective cesarean sections.
Obstetric anesthesia care accounts for approximately 12% of the American Society of Anesthesiologists (ASA) Closed Claims database claims. A comparison of obstetric anesthesia claims from 1990 to 2003 or with pre-1990 claims shows a decrease in maternal deaths, as well as a decrease in respiratory-damaging events (aspiration, difficult intubation, esophageal intubation, and inadequate oxygenation/ventilation). Although newborn deaths and brain damage also decreased over this period, they remained a leading cause of obstetric anesthesia malpractice claims. Maternal nerve injury was more common in claims reported after 1990 compared with earlier years.
General Approach to the Obstetric Patient
All patients entering the obstetric suite potentially require anesthesia services, whether planned or emergent. Patients requiring anesthetic care for labor or cesarean section should undergo a focused preanesthetic evaluation as early as possible. This should consist of a maternal health history, anesthesia and anesthesia-related obstetric history, blood pressure measurement, airway assessment, and back examination for regional anesthesia.
 Regardless of the time of last oral intake, all patients are considered to have a full stomach and to be at risk for pulmonary aspiration. Because the duration of labor is often prolonged, guidelines usually allow small amounts of oral clear liquid for uncomplicated labor. The minimum fasting period for elective cesarean section remains controversial, but is recommended to be 6 h for light meals and 8 h for heavy meals. Prophylactic administration of a clear antacid (15-30 mL of 0.3 M sodium citrate orally) every 30 min prior to a cesarean section can help maintain gastric pH greater than 2.5 and may decrease the likelihood of severe aspiration pneumonitis. An H2-blocking drug (ranitidine, 100-150 mg orally or 50 mg intravenously) or metoclopramide, 10 mg orally or intravenously, should also be considered in high-risk patients and in those expected to receive general anesthesia. H2 blockers reduce both gastric volume and pH but have no effect on the gastric contents already present. Metoclopramide accelerates gastric emptying, decreases gastric volume, and increases lower esophageal sphincter tone. The supine position should be avoided unless a left uterine displacement device (>15° wedge) is placed under the right hip.
Regardless of the time of last oral intake, all patients are considered to have a full stomach and to be at risk for pulmonary aspiration. Because the duration of labor is often prolonged, guidelines usually allow small amounts of oral clear liquid for uncomplicated labor. The minimum fasting period for elective cesarean section remains controversial, but is recommended to be 6 h for light meals and 8 h for heavy meals. Prophylactic administration of a clear antacid (15-30 mL of 0.3 M sodium citrate orally) every 30 min prior to a cesarean section can help maintain gastric pH greater than 2.5 and may decrease the likelihood of severe aspiration pneumonitis. An H2-blocking drug (ranitidine, 100-150 mg orally or 50 mg intravenously) or metoclopramide, 10 mg orally or intravenously, should also be considered in high-risk patients and in those expected to receive general anesthesia. H2 blockers reduce both gastric volume and pH but have no effect on the gastric contents already present. Metoclopramide accelerates gastric emptying, decreases gastric volume, and increases lower esophageal sphincter tone. The supine position should be avoided unless a left uterine displacement device (>15° wedge) is placed under the right hip.
Anesthesia for Labor & Vaginal Delivery
The pain of labor arises from contraction of the myometrium against the resistance of the cervix and perineum, progressive dilation of the cervix and lower uterine segment, and stretching and compression of pelvic and perineal structures.
Pain during the first stage of labor is primarily visceral pain resulting from uterine contractions and cervical dilation. It is usually initially confined to the T11-T12 dermatomes during the latent phase, but eventually involves the T10-L1 dermatomes as labor enters the active phase. The visceral afferent fibers responsible for labor pain travel with sympathetic nerve fibers first to the uterine and cervical plexuses, then through the hypogastric and aortic plexuses, before entering the spinal cord with the T10-L1 nerve roots. The pain is initially perceived in the lower abdomen but may increasingly be referred to the lumbosacral area, gluteal region, and thighs as labor progresses. Pain intensity also increases with progressive cervical dilation and with increasing intensity and frequency of uterine contractions. Nulliparous women and those with a history of dysmenorrhea appear to experience greater pain during the first stage of labor.
The onset of perineal pain at the end of the first stage signals the beginning of fetal descent and the second stage of labor. Stretching and compression of pelvic and perineal structures intensifies the pain. Sensory innervation of the perineum is provided by the pudendal nerve (S2-4) so pain during the second stage of labor involves the T10-S4 dermatomes.
Psychological and nonpharmacological techniques are based on the premise that the pain of labor can be suppressed by reorganizing one’s thoughts. Patient education and positive conditioning about the birthing process are central to such techniques. Pain during labor tends to be accentuated by fear of the unknown or previous unpleasant experiences. Techniques include those of Bradley, Dick-Read, Lamaze, and LeBoyer. The Lamaze technique, one of the most popular, coaches the parturient to take a deep breath at the beginning of each contraction followed by rapid, shallow breathing for the duration of the contraction. The parturient also concentrates on an object in the room and attempts to focus her thoughts away from the pain. Less common nonpharmacological techniques include hypnosis, transcutaneous electrical nerve stimulation, biofeedback, and acupuncture. The success of all these techniques varies considerably from patient to patient, and many patients require additional forms of analgesia.
 Nearly all parenteral opioid analgesics and sedatives readily cross the placenta and can affect the fetus. Concern over fetal depression limits the use of these agents to the early stages of labor or to situations in which regional anesthetic techniques are not available or appropriate. Central nervous system depression in the neonate may be manifested by a prolonged time to sustain respirations, respiratory acidosis, or an abnormal neurobehavioral examination. Moreover, loss of beat-to-beat variability in the fetal heart rate (seen with most central nervous system depressants) and decreased fetal movements (due to sedation of the fetus) complicate the evaluation of fetal well-being during labor. Long-term fetal heart rate variability is affected more than short-term variability. The degree and significance of these effects depend on the specific agent, the dose, the time elapsed between its administration and delivery, and fetal maturity. Premature neonates exhibit the greatest sensitivity. In addition to maternal respiratory depression, opioids can also induce maternal nausea and vomiting and delay gastric emptying. Some clinicians have advocated use of opioids via patient-controlled analgesia (PCA) devices early in labor because this technique appears to reduce total opioid requirements.
Nearly all parenteral opioid analgesics and sedatives readily cross the placenta and can affect the fetus. Concern over fetal depression limits the use of these agents to the early stages of labor or to situations in which regional anesthetic techniques are not available or appropriate. Central nervous system depression in the neonate may be manifested by a prolonged time to sustain respirations, respiratory acidosis, or an abnormal neurobehavioral examination. Moreover, loss of beat-to-beat variability in the fetal heart rate (seen with most central nervous system depressants) and decreased fetal movements (due to sedation of the fetus) complicate the evaluation of fetal well-being during labor. Long-term fetal heart rate variability is affected more than short-term variability. The degree and significance of these effects depend on the specific agent, the dose, the time elapsed between its administration and delivery, and fetal maturity. Premature neonates exhibit the greatest sensitivity. In addition to maternal respiratory depression, opioids can also induce maternal nausea and vomiting and delay gastric emptying. Some clinicians have advocated use of opioids via patient-controlled analgesia (PCA) devices early in labor because this technique appears to reduce total opioid requirements.
Meperidine, a commonly used opioid, can be given in doses of 10-25 mg intravenously or 25-50 mg intramuscularly, usually up to a total of 100 mg. Maximal maternal and fetal respiratory depression is seen in 10-20 min following intravenous administration and in 1-3 h following intramuscular administration. Consequently, meperidine is usually administered early in labor when delivery is not expected for at least 4 h. Intravenous fentanyl, 25-100 mcg/h, has also been used for labor. Fentanyl in 25-100 mcg doses has a 3- to 10-min analgesic onset that initially lasts about 60 min, and lasts longer following multiple doses. However, maternal respiratory depression outlasts the analgesia. Lower doses of fentanyl may be associated with little or no neonatal respiratory depression and are reported to have no effect on Apgar scores. Morphine is not used because in equianalgesic doses it appears to cause greater respiratory depression in the fetus than meperidine and fentanyl. Agents with mixed agonist-antagonist activity (butorphanol, 1-2 mg, and nalbuphine, 10-20 mg intravenously or intramuscularly) are effective and are associated with little or no cumulative respiratory depression, but excessive sedation with repeat doses can be problematic.
Promethazine (25-50 mg intramuscularly) and hydroxyzine (50-100 mg intramuscularly) can be useful alone or in combination with meperidine. Both drugs reduce anxiety, opioid requirements, and the incidence of nausea, but do not add appreciably to neonatal depression. A significant disadvantage of hydroxyzine is pain at the injection site following intramuscular administration. Nonsteroidal antiinflammatory agents, such as ketorolac, are not recommended because they suppress uterine contractions and promote closure of the fetal ductus arteriosus.
Small doses (up to 2 mg) of midazolam (Versed) may be administered in combination with a small dose of fentanyl (up to 100 mcg) in healthy parturients at term to facilitate neuraxial blockade. At this dose, maternal amnesia has not been observed. Chronic administration of the longer-acting benzodiazepine diazepam (Valium) has been associated with fetal depression.
Low-dose intravenous ketamine is a powerful analgesic. In doses of 10-15 mg intravenously, good analgesia can be obtained in 2-5 min without loss of consciousness. Unfortunately, fetal depression with low Apgar scores is associated with doses greater than 1 mg/kg. Large boluses of ketamine (>1 mg/kg) can be associated with hypertonic uterine contractions. Low-dose ketamine is most useful just prior to delivery or as an adjuvant to regional anesthesia. Some clinicians avoid use of ketamine because it may produce unpleasant psychotomimetic effects (see Chapter 9).
In the past, reduced concentrations of volatile anesthetic agents (eg, methoxyflurane) in oxygen were sometimes used for relief of milder labor pain. Inhalation of nitrous oxide-oxygen remains in common use for relief of mild labor pain in many countries. As previously noted, nitrous oxide has minimal effects on uterine blood flow or uterine contractions.
Pudendal nerve blocks are often combined with perineal infiltration of local anesthetic to provide perineal anesthesia during the second stage of labor when other forms of anesthesia are not employed or prove to be inadequate. Paracervical plexus blocks are no longer used because of their association with a relatively high rate of fetal bradycardia; the close proximity of the injection site to the uterine artery may result in uterine arterial vasoconstriction, uteroplacental insufficiency, and increased levels of the local anesthetic in the fetal blood.
During a pudendal nerve block, a special needle (Koback) or guide (Iowa trumpet) is used to place the needle transvaginally underneath the ischial spine on each side (see Chapter 48); the needle is advanced 1-1.5 cm through the sacrospinous ligament, and 10 mL of 1% lidocaine or 2% chloroprocaine is injected following aspiration. The needle guide is used to limit the depth of injection and protect the fetus and vagina from the needle. Other potential complications include intravascular injection, retroperitoneal hematoma, and retropsoas or subgluteal abscess.
Epidural or intrathecal techniques, alone or in combination, are currently the most popular methods of pain relief during labor and delivery. They can provide excellent analgesia while allowing the mother to be awake and cooperative during labor. Although spinal opioids or local anesthetics alone can provide satisfactory analgesia, techniques that combine the two have proved to be the most satisfactory in most parturients.  Moreover, the synergy between opioids and local anesthetics decreases dose requirements and provides excellent analgesia with few maternal side effects and little or no neonatal depression.
Moreover, the synergy between opioids and local anesthetics decreases dose requirements and provides excellent analgesia with few maternal side effects and little or no neonatal depression.
Opioids may be given intrathecally as a single injection or intermittently via an epidural or intrathecal catheter (Table 41-2). Relatively large doses are required for analgesia during labor when epidural or intrathecal opioids are used alone. For example, the ED50 during labor is 124 mcg for epidural fentanyl and 21 mcg for epidural sufentanil. The higher doses may be associated with a high risk of side effects, most importantly respiratory depression. For that reason combinations of local anesthetics and opioids are most commonly used (see below). Pure opioid techniques are most useful for high-risk patients who may not tolerate the functional sympathectomy associated with spinal or epidural anesthesia (see Chapter 45). This group includes patients with hypovolemia or significant cardiovascular disease such as moderate to severe aortic stenosis, tetralogy of Fallot, Eisenmenger’s syndrome, or pulmonary hypertension. With the exception of meperidine, which has local anesthetic properties, spinal opioids alone do not produce motor blockade or sympathectomy. Thus, they do not impair the ability of the parturient to “push.” Disadvantages include less complete analgesia, lack of perineal relaxation, and side effects such as pruritus, nausea, vomiting, sedation, and respiratory depression. Side effects may be ameliorated with low doses of naloxone (0.1-0.2 mg/h intravenously).
Intrathecal morphine in doses of 0.1-0.5 mg may produce satisfactory and prolonged (4-6 h) analgesia during the first stage of labor. Unfortunately, the onset of analgesia is slow (45-60 min), and these doses may not be sufficient in many patients. Higher doses are associated with a relatively high incidence of side effects. Morphine is therefore rarely used alone. The combination of morphine, 0.1-0.25 mg, and fentanyl, 12.5 mcg (or sufentanil, 5 mcg), may result in a more rapid onset of analgesia (5 min). Intermittent boluses of 10-15 mg of meperidine, 12.5-25 mcg of fentanyl, or 3-10 mcg of sufentanil via an intrathecal catheter can also provide satisfactory analgesia for labor. Early reports of fetal bradycardia following intrathecal opioid injections (eg, sufentanil) have not been confirmed by subsequent studies. Hypotension following administration of intrathecal opioids for labor is likely related to the resultant analgesia and decreased circulating catecholamine levels.
Relatively large doses (≥7.5 mg) of epidural morphine are required for satisfactory labor analgesia, but doses larger than 5 mg are not recommended because of the increased risk of delayed respiratory depression and because the resultant analgesia is effective only in the early first stage of labor. Onset may take 30-60 min but analgesia lasts up to 12-24 h (as does the risk of delayed respiratory depression). Epidural meperidine, 50-100 mg, provides good, but relatively brief, analgesia (1-3 h). Epidural fentanyl, 50-150 mcg, or sufentanil, 10-20 mcg, usually produces analgesia within 5-10 min with few side effects, but it has a short duration (1-2 h). Although “single-shot” epidural opioids do not appear to cause significant neonatal depression, caution should be exercised following repeated administrations. Combinations of a lower dose of morphine, 2.5 mg, with fentanyl, 25-50 mcg (or sufentanil, 7.5-10 mcg), may result in a more rapid onset and prolongation of analgesia (4-5 h) with fewer side effects.
Epidural and spinal (intrathecal) analgesia more commonly utilizes local anesthetics either alone or with opioids for labor and delivery.  Analgesia during the first stage of labor requires neural blockade at the T10-L1 sensory level, whereas pain relief during the second stage of labor requires neural blockade at T10-S4.
Analgesia during the first stage of labor requires neural blockade at the T10-L1 sensory level, whereas pain relief during the second stage of labor requires neural blockade at T10-S4.  Continuous lumbar epidural analgesia is the most versatile and most commonly-employed technique, because it can be used for pain relief for the first stage of labor as well as analgesia/anesthesia for subsequent vaginal delivery or cesarean section, if necessary. “Single-shot” epidural, spinal, or combined spinal epidural analgesia may be appropriate when pain relief is initiated just prior to vaginal delivery (the second stage). Obstetric caudal injections have largely been abandoned because of less versatility; although effective for perineal analgesia/anesthesia they require large volumes of local anesthetic to anesthetize upper lumbar and lower thoracic dermatomes. They have also been associated with early paralysis of the pelvic muscles that may interfere with normal rotation of the fetal head, and with a small risk of accidental puncture of the fetus.
Continuous lumbar epidural analgesia is the most versatile and most commonly-employed technique, because it can be used for pain relief for the first stage of labor as well as analgesia/anesthesia for subsequent vaginal delivery or cesarean section, if necessary. “Single-shot” epidural, spinal, or combined spinal epidural analgesia may be appropriate when pain relief is initiated just prior to vaginal delivery (the second stage). Obstetric caudal injections have largely been abandoned because of less versatility; although effective for perineal analgesia/anesthesia they require large volumes of local anesthetic to anesthetize upper lumbar and lower thoracic dermatomes. They have also been associated with early paralysis of the pelvic muscles that may interfere with normal rotation of the fetal head, and with a small risk of accidental puncture of the fetus.
Absolute contraindications to regional anesthesia include patient refusal, infection over the injection site, coagulopathy, marked hypovolemia, and true allergies to local anesthetics. The patient’s inability to cooperate may prevent successful regional anesthesia. Neuraxial anesthesia and full anticoagulation is a dangerous combination. Regional anesthesia should generally not be performed within 6-8 h of a subcutaneous minidose of unfractionated heparin or within 12-24 h of administration of low-molecular-weight heparin (LMWH). Thrombocytopenia or concomitant administration of an antiplatelet agent increases the risk of spinal hematoma. A vaginal birth after cesarean (VBAC) delivery is not considered a contraindication to regional anesthesia during labor. Concern that the anesthesia may mask pain associated with uterine rupture during VBAC may not be justified, because dehiscence of a lower segment scar frequently does not cause pain even without epidural anesthesia; moreover, changes in uterine tone and contraction pattern may be more reliable signs.
Before performing any regional block, appropriate equipment and supplies for resuscitation should be checked and made immediately available. Minimum supplies include oxygen, suction, a mask with a positive-pressure device for ventilation, a functioning laryngoscope and blades, endotracheal tubes (6 or 6.5 mm), oral and nasal airways, intravenous fluids, ephedrine, atropine, propofol, and succinylcholine. The ability to frequently monitor blood pressure and heart rate is mandatory. A pulse oximeter and capnograph should be readily available.
Epidural analgesia for labor may be administered in early labor after the patient has been evaluated by her obstetrician.  When dilute mixtures of a local anesthetic and an opioid are used, epidural analgesia has little if any effect on the progress of labor. Concerns that regional analgesia will increase the likelihood of oxytocin augmentation, operative (eg, forceps) delivery, or cesarean section, are unjustified. It is often advantageous to place an epidural catheter early, when the patient is less uncomfortable and can be positioned more easily. Moreover, should an urgent or emergent cesarean section become necessary, the presence of a well-functioning epidural catheter makes it possible to avoid general anesthesia.
When dilute mixtures of a local anesthetic and an opioid are used, epidural analgesia has little if any effect on the progress of labor. Concerns that regional analgesia will increase the likelihood of oxytocin augmentation, operative (eg, forceps) delivery, or cesarean section, are unjustified. It is often advantageous to place an epidural catheter early, when the patient is less uncomfortable and can be positioned more easily. Moreover, should an urgent or emergent cesarean section become necessary, the presence of a well-functioning epidural catheter makes it possible to avoid general anesthesia.
Parturients may be positioned on their sides or in the sitting position for the procedure. The sitting position often makes it easier to identify the midline and spine in obese patients. When epidural anesthesia is being given for vaginal delivery (second stage), the sitting position helps ensure good sacral spread.
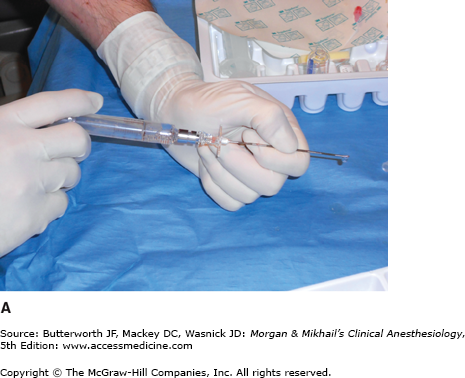
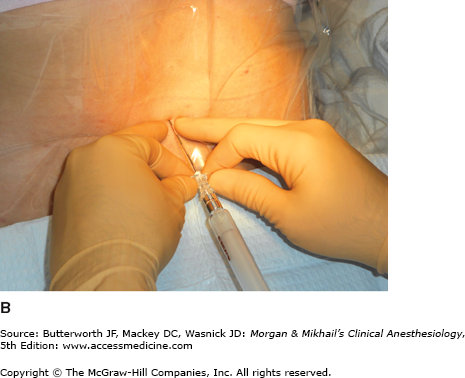
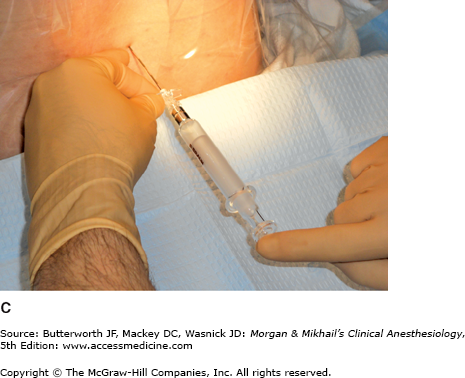
Because the lumbar epidural space pressure may be positive in some parturients, correct identification of the epidural space may be difficult. Unintentional dural puncture will occur even in experienced hands; the incidence of “wet taps” in obstetric patients is 0.25-9%, depending on clinician experience. Many practitioners add a compressible air bubble to the saline syringe and bounce the plunger to ensure that it moves freely and does not stick to the syringe wall (Figure 41-1A and C). Most clinicians advocate the midline approach, whereas a minority favors the paramedian approach. For the placement of a lumbar epidural catheter in the obstetric patient, most anesthesiologists advance the epidural needle with the left hand, which is braced against the patient’s back, while applying continuous pressure to a glass syringe filled with sterile saline (Figure 41-1A and C). Alternatively, some make use of the “wings” of the Weiss epidural needle by advancing it with both hands few millimeters at a time (Figure 41-1B). A change of tissue resistance is then tested continuously using tactile feedback when advancing the needle and by intermittently applying pressure to the air-filled loss-of resistance syringe. The later technique allows for precise control of needle advancement and may allow a better distinction of various tissue densities. If air is used for detecting loss of resistance, the amount injected should be limited; injection of larger volumes of air (>2-3 mL) in the epidural space has been associated with patchy or unilateral analgesia and headache. The average depth of the lumbar epidural space in obstetric patients is reported to be 5 cm from the skin. Placement of the epidural catheter at the L3-4 or L4-5 interspace is generally optimal for achieving a T10-S5 neural blockade. Ultrasound guidance has recently been offered as tool in assisting with the placement of an epidural catheter. This technique allows the practitioner to judge the depth of the epidural space and estimate the best angle of needle insertion. The potential benefit of this technique is most obvious in obese patients with poor anatomic landmarks. However, the technique is highly user-dependent, and few practitioners have adopted it.
Figure 41-1
A: One-handed needle advancement; continuous pressure technique. The operator applies continuous pressure to the plunger of a loss-of-resistance syringe filled with saline and an air bubble while advancing the needle with the left hand braced against the patient’s back. B: Bimanual needle advancement; intermittent pressure technique. The operator advances the loss-of-resistance syringe with both hands 2-3 mm at a time while appreciating the resistance encountered by the needle. C: In between bimanual advancements of the needle, the operator tests the tissue resistance of the needle tip by bouncing the plunger of the air-filled loss-of-resistance syringe. Many practitioners add a compressible air bubble to a saline-filled syringe and bounce the plunger to ensure that the plunger is moving freely and not sticking to the syringe barrel wall.
If unintentional dural puncture occurs, the anesthetist has two choices: (1) place the epidural catheter in the subarachnoid space for continuous spinal (intrathecal) analgesia and anesthesia (see below), or (2) remove the needle and attempt placement at a higher spinal level. The intrathecally-placed epidural catheter may be used as continuous spinal anesthetic, possibly reducing the incidence of post-dural puncture headache. If used in this fashion, an infusion of 0.0625-0.125% bupivacaine with fentanyl, 2-3 mcg/mL starting at 1-3 mL/h, is a reasonable choice.
Many clinicians advocate use of a multiholed catheter instead of a single-holed catheter for obstetric anesthesia. Use of a multiholed catheter may be associated with fewer unilateral blocks and greatly reduces the incidence of false-negative aspiration when assessing for intravascular or intrathecal catheter placement. Advancing a multiholed catheter 4-6 cm into the epidural space appears to be optimal for obtaining adequate sensory levels. A single-hole catheter need only be advanced 3-5 cm into the epidural space. Shorter insertion depths (<5 cm), however, may favor dislodgment of the catheter out of the epidural space in obese patients following flexion/ extension movements of the spine. Spiral wire-reinforced catheters are very resistant to kinking. A spiral or spring tip, particularly when used without a stylet, is associated with fewer, less intense paresthesias and may also be associated with a lower incidence of accidental intravascular insertion.
The addition of opioids to local anesthetic solutions for epidural anesthesia has dramatically changed the practice of obstetric anesthesia. The synergy between epidural opioids and local anesthetic solutions reflects separate sites of action, namely, opiate receptors and neuronal axons, respectively. When the two are combined, very low concentrations of both local anesthetic and opioid can be used. More importantly, the incidence of adverse side effects, such as hypotension and drug toxicity, is likely reduced. Although local anesthetics can be used alone, there is rarely a reason to do so. Moreover, when an opioid is omitted, the higher concentration of local anesthetic required (eg, bupivacaine, 0.25%, and ropivacaine, 0.2%) for adequate analgesia can impair the parturient’s ability to push effectively as labor progresses. Bupivacaine or ropivacaine in concentrations of 0.0625-0.125% with either fentanyl, 2-3 mcg/mL, or sufentanil, 0.3-0.5 mcg/mL, is most often used. In general, the lower the concentration of the local anesthetic the greater the concentration of opioid that is required. Very dilute local anesthetic mixtures (0.0625%) generally do not produce motor blockade and may allow some patients to ambulate (“walking” or “mobile” epidural). The long duration of action of bupivacaine makes it a popular agent for labor. Ropivacaine may be preferable because of its reduced potential for cardiotoxicity (see Chapter 16). At equi-analgesic doses, ropivacaine and bupivacaine appear to produce the same degree of motor block.
The effect of epinephrine-containing solutions on the course of labor is somewhat controversial. Many clinicians use epinephrine-containing solutions only for intravascular test doses because of concern that the solutions may slow the progression of labor or adversely affect the fetus; others use only very dilute concentrations of epinephrine such as 1:800,000 or 1:400,000. Studies comparing these various agents have failed to find any differences in neonatal Apgar scores, acid-base status, or neurobehavioral evaluations.
Initial epidural injections may be done either before or after the catheter is placed. Administration through the needle can facilitate catheter placement, whereas administration through the catheter ensures proper function of the catheter. The following sequence is suggested for epidural activation:
Test for unintentional subarachnoid or intravascular placement of the needle or catheter with a 3-mL test dose of a local anesthetic with 1:200,000 epinephrine (controversial; see the section on Prevention of Unintentional Intravascular and Intrathecal Injections). Many clinicians test with lidocaine 1.5% because of less toxicity following unintentional intravascular injection and a more rapid onset of spinal anesthesia than with bupivacaine and ropivacaine. The test dose should be injected between contractions to help reduce false positive signs of an intravascular injection (ie, tachycardia due to a painful contraction).
If after 5 min signs of intravascular or intrathecal injection are absent, with the patient supine and left uterine displacement, administer 10 mL of the local anesthetic-opioid mixture in 5-mL increments, waiting 1-2 min between doses, to achieve a T10-L1 sensory level. The initial bolus is usually composed of 0.1-0.2% ropivacaine or 0.0625-0.125% bupivacaine combined with either 50-100 mcg of fentanyl or 10-20 mcg of sufentanil.
Monitor with frequent blood pressure measurements for 20-30 min or until the patient is stable. Pulse oximetry should also be used. Oxygen is administered via face mask if there are any significant decreases in blood pressure or oxygen saturation readings.
Repeat steps 2 and 3 when pain recurs until the first stage of labor is completed. Alternatively, a continuous epidural infusion technique may be employed using bupivacaine or ropivacaine in concentrations of 0.0625-0.125% with either fentanyl, 1-5 mcg/mL, or sufentanil, 0.2-0.5 mcg/mL at a rate of 10 mL/h, which subsequently is adjusted to the patient’s analgesic requirements (range: 5-15 mL/h). A third choice would be to use patient-controlled epidural analgesia (PCEA). Some studies suggest that total drug requirements may be less and patient satisfaction is greater with PCEA compared with other epidural techniques. PCEA settings are typically a 5-mL bolus dose with a 5-10 min lockout and 0-12 mL/h basal rate; a 1-h limit of 15-25 mL may used. Migration of the epidural catheter into a blood vessel during a continuous infusion technique may be heralded by loss of effective analgesia; a high index of suspicion is required because overt signs of systemic toxicity may be absent. Erosion of the catheter through the dura results in a slowly progressive motor blockade of the lower extremities and a rising sensory level.
Administration for the second stage of labor extends the block to include the S2-4 dermatomes. Whether a catheter is already in place or epidural anesthesia is just being initiated, the following steps should be undertaken:
If the patient does not already have a catheter in place, identify the epidural space while the patient is in a sitting position. A patient who already has an epidural catheter in place should be placed in a semiupright or sitting position prior to injection.
Give a 3-mL test dose of local anesthetic (eg, lidocaine 1.5%) with 1:200,000 epinephrine. Again, the injection should be completed between contractions.
If after 5 min signs of an intravascular or intrathecal injection are absent, give 10-15 mL of additional local anesthetic-opioid mixture at a rate not faster than 5 mL every 1-2 min.
Administer oxygen by face mask, lay the patient supine with left uterine displacement, and monitor blood pressure every 1-2 min for the first 15 min, then every 5 min thereafter.
Safe administration of epidural anesthesia is critically dependent on avoiding unintentional intrathecal or intravascular injection.  Unintentional intravascular or intrathecal placement of an epidural needle or catheter is possible even when aspiration fails to yield blood or cerebrospinal fluid (CSF). The incidence of unintentional intravascular or intrathecal placement of an epidural catheter is 5-15% and 0.5-2.5%, respectively. Even a properly placed catheter can subsequently erode into an epidural vein or an intrathecal position. This possibility should be considered each time local anesthetic is injected through an epidural catheter.
Unintentional intravascular or intrathecal placement of an epidural needle or catheter is possible even when aspiration fails to yield blood or cerebrospinal fluid (CSF). The incidence of unintentional intravascular or intrathecal placement of an epidural catheter is 5-15% and 0.5-2.5%, respectively. Even a properly placed catheter can subsequently erode into an epidural vein or an intrathecal position. This possibility should be considered each time local anesthetic is injected through an epidural catheter.
Test doses of lidocaine, 45-60 mg, bupivacaine, 7.5-10 mg, ropivacaine, 6-8 mg, or chloroprocaine, 100 mg, can be given to exclude unintentional intrathecal placement. Signs of sensory and motor blockade usually become apparent within 2-3 min and 3-5 min, respectively, if the injection is intrathecal.
In patients not receiving β-adrenergic antagonists, the intravascular injection of a local anesthetic solution with 15-20 mcg of epinephrine consistently increases the heart rate by 20-30 beats/min within 30-60 s if the catheter (or epidural needle) is intravascular. This technique is not always reliable in parturients because they often have marked spontaneous baseline variations in heart rate with contractions. In fact, bradycardia has been reported in a parturient following intravenous injection of 15 mcg of epinephrine. Moreover, in animal studies, 15 mcg of epinephrine intravenously reduces uterine blood flow. Alternative methods of detecting unintentional intravascular catheter placement include eliciting tinnitus or perioral numbness following a 100-mg test dose of lidocaine or eliciting a chronotropic effect following injection of 5 mcg of isoproterenol. The use of dilute local anesthetic solutions and slow injection rates of no more than 5 mL at a time may also enhance detection of unintentional intravascular injections before catastrophic complications develop.
Generally defined as a greater than 20% decrease in the patient’s baseline blood pressure, or a systolic blood pressure less than 100 mm Hg, hypotension is a common side effect of neuraxial anesthesia. It is primarily due to decreased sympathetic tone and is greatly accentuated by aortocaval compression and an upright or semiupright position. Treatment should be aggressive in obstetric patients and consists of intravenous boluses of ephedrine (5-15 mg) or phenylephrine (25-50 mcg), supplemental oxygen, left uterine displacement, and an intravenous fluid bolus. Although the routine use of a crystalloid fluid bolus prior to dosing an epidural catheter is not effective in the prevention of hypotension, ensuring proper intravenous hydration of the pregnant patient is important. Use of the head-down (Trendelenburg) position is controversial because of its potentially detrimental effects on pulmonary gas exchange.
Early recognition of intravascular injection, facilitated by the use of small, repeated doses of local anesthetic instead of a large bolus, may prevent more serious local anesthetic toxicity, such as seizures or cardiovascular collapse. Intravascular injections of toxic doses of lidocaine or chloroprocaine usually present as seizures. Propofol, 20-50 mg, will terminate seizure activity. Maintenance of a patent airway and adequate oxygenation are critical; however, immediate endotracheal intubation with succinylcholine and cricoid pressure is rarely necessary. Intravascular injections of bupivacaine can cause rapid and profound cardiovascular collapse as well as seizure activity. Cardiac resuscitation may be exceedingly difficult and is aggravated by acidosis and hypoxia. An immediate infusion of 20% Intralipid has shown efficacy in reversing bupivacaine-induced cardiac toxicity. Amiodarone is the agent of choice for treating local anesthetic-induced ventricular arrhythmias.
Even when dural puncture is recognized immediately after injection of local anesthetic, attempted aspiration of the local anesthetic will usually be unsuccessful. The patient should be placed supine with left uterine displacement. Head elevation accentuates the adverse cerebral effects of hypotension and should be avoided. Hypotension should be treated with phenylephrine and intravenous fluids. A high spinal level can also result in diaphragmatic paralysis, which necessitates intubation and ventilation with 100% oxygen. Delayed onset of a very high and often patchy or unilateral block may be due to unrecognized subdural injection (see Chapter 45), which is managed similarly.
Headache frequently follows unintentional dural puncture in parturients. A self-limited headache may occur without dural puncture; in such instances, injection of significant amounts of air into the epidural space during a loss-of-resistance technique may be responsible. PDPH is due to decreased intracranial pressure with compensatory cerebral vasodilation (see Chapter 45). Bed rest, hydration, oral analgesics, and caffeine sodium benzoate (500 mg added to 1000 mL intravenous fluids administered at 200 mL/h) may be effective in patients with mild headaches and as temporary treatment. Patients with moderate to severe headaches usually require an epidural blood patch (10-20 mL) (see Chapter 45). Prophylactic epidural blood patches are not recommended; 25-50% of patients may not require a blood patch following dural puncture. Delaying a blood patch for 24 h increases its efficacy. Intracranial subdural hematoma has been reported as a rare complication 1-6 weeks following unintentional dural puncture in obstetric patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 Hypotension
Hypotension 

