Screening parameters
Diagnostic procedure
TTE
TEE (3D-TEE)
CT
R/L catheter
Severity of the aortic valve stenosis
++
++
+
++
Fixated vs. functional stenosis
++ (Dobutamine)
−
−
Aortic valve anatomy and morphology
+
++
++
±
Aortic annulus
−
+
++
±
Vascular access
−
−
++
+
Catheter passage
−
±
++
+
Coronary heart disease
−
−
±
++
Aortic Valve Anatomy and Morphology
Precise measurement of the aortic annulus is decisive for transcatheter heart valve (THV) size selection. Due to the complex, frequently oval anatomy of the aortic annulus, three-dimensional transesophageal echocardiography (3D-TEE) and/or computed tomography (CT) do provide decisive additional information and should be used in supplement to transthoracic echocardiography (TTE) and/or angiography [11, 12]. Minimal, maximal, and the annulus area derived diameter help to understand the annulus anatomy and enable an optimal prosthesis selection [13]. Moreover, the assessment of aortic valve anatomy and the severity as well as localization of the aortic valve calcification are important components of TAVR screening. Due to the danger of an implantation-associated aortic rupture, a bicuspid aortic valve is a typical contraindication, which must be reliably ruled out during screening [14].
Aortic Root and Coronary Ostia Distance
Assessment of the aortic root is particularly important for implantation of the self-expandable Medtronic (Minneapolis, MN) CoreValve® System. Because of the directional and fixation mechanism of the prosthesis, extremely narrow or broad aortic roots are contraindications. These limitations do not apply to the balloon-expandable Edwards THVs. Measurements of the aortic annulus, the sinus of Valsalva, and the ascending aorta are necessary. CT and MRI are considered the gold standard as a result of the potential for multiplane reconstruction with orthogonal measurement in all segments [15]. Both procedures also enable a thorough assessment of the degree of calcification of the aortic root and may therefore be helpful in selecting the path of access. Moreover, in assessing the risk of the rare but often fatal coronary ostia obstruction, determination of the spacing of coronary orifices at the level of the aortic valve is essential [16].
Vascular Access and Catheter Passage
In planning the vascular access, evaluation of the size, the degree of calcification, and the vascular tortuosity of the peripheral arteries and of the abdominal and thoracic aorta is essential [17]. The coincidence of symptomatic aortic valve stenosis and peripheral vascular disease is known and increases the risk of complications [18]. Therefore, a transapical procedure should be preferred in the case of small-caliber femoral arteries (<5.5–6 mm), extremely calcified and tortuous iliacal or femoral vessels, or in the presence of a porcelain aorta [16]. In addition to the invasive angiography, CT and MRI are available for the assessment. The latter not only enable measurement of the endoluminal diameter but also provide information for determination of the extent and site of calcifications and stenosis.
Supplemental Diagnostics
In addition to the examination procedures already listed, TAVR screening is completed by right/left coronary catheter (including AOA determination, coronary angiography, bulbus and iliacal/femoral artery angiography), lung function testing, and laboratory tests. Important information on accompanying diseases of the heart, lungs, kidneys, or liver can thus be collected and used for therapy decisions for the individual patient. Serious secondary findings are not infrequently disclosed which may in part explain the patient’s symptoms (e.g., coronary heart disease as a cause of dyspnoea), and sometimes require re-evaluation of the life expectancy and indication for TAVR (e.g., neoplasia of the pancreas).
Implantation Technique
The basic principle of the procedure is the percutaneous, transcatheter implantation of a bioprosthesis anchored in a metal frame (THV). After balloon aortic valvuloplastie or immediately, the THV affixed to the tip of the catheter is positioned retrograde or anterograde in the aortic annulus of the native aortic valve and is then expanded. To date, there are primarily two types of transcatheter aortic valve prostheses in broad clinical use: the balloon-expandable Edwards-Bioprosthesis (Edwards Lifesciences Corporation, Irvine, CA) and the self-expanding CoreValve System (Medtronic Inc., Irvine, CA). The technical details and anatomic prerequisites of both are summarized in Table 27.2.
Table 27.2
Technical details and anatomic prerequisites of the Edwards SAPIEN XT® (Edwards Lifesciences, Irvine, CA) and Medtronic CoreValve® prosthesis (Medtronic, Minneapolis, MN)
Edwards SAPIEN XT THV | Medtronic CoreValve | |||||
|---|---|---|---|---|---|---|
Technical details | Cobalt chromium stent | Nitinol frame | ||||
Tricuspid bovine pericardial valve | Tricuspid porcine pericardial valve | |||||
Prosthesis | ||||||
Size (mm) | 23 | 26 | 29 | 26 | 29 | 31 |
Catheter system | ||||||
Transfemoral (Fr) | 16a | 18 | 20 | 18 | 18 | 18 |
Transapical (Fr) | 24 | 24 | 26 | – | – | – |
Anatomic prerequisites | ||||||
Aortic annulus (mm) | 18–22 | 21–25 | 24–27 | 20–23 | 23–27 | 26–29 |
Coronary ostia distance (mm) | ≥10 | ≥10 | ≥10 | – | – | – |
Height of the sinus of Valsalva (mm) | – | – | – | ≥15 | ≥15 | ≥15 |
Vascular diameter of catheter passage (mm) | ≥6 | ≥6.5 | ≥7 | ≥6 | ≥6 | ≥6 |
Balloon-Expandable Bioprostheses
The current generation of the Edwards-THV, the SAPIEN XT® (Edwards Lifesciences Corporation, Irvine, CA) (Fig. 27.1a), consists of a cobalt-chrome frame with integrated tricuspid bovine pericardial valve. Analogous to coronary stent implantation, implantation of the prosthesis is performed by balloon inflation. A high-frequency, right ventricular stimulation (180–220 bpm) for minimizing the transaortic flow stabilizes the prosthesis during the expansion phase and prevents prosthesis dislocation. The SAPIEN XT® is available in three sizes (23, 26, and 29 mm) and can be used in aortic annuli from 20 to 27 mm. Currently, the third generation THV (SAPIEN 3®, Fig. 27.1b) is just being introduced in Europe. It is mainly characterized by a new frame design, intended for a lower delivery profile, and an outer skirt designed to reduce paravalvular leakage. The first two generations of the Edwards-Bioprosthesis (Cribier-Edwards and Edwards SAPIEN®) were tested in randomized studies for transfemoral and transapical procedures.
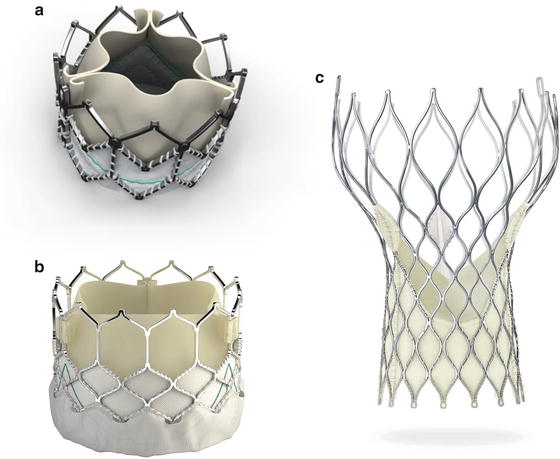

Fig. 27.1
(a, b) Balloon-expandable Edwards SAPIEN XT® and SAPIEN 3® THV (Courtesy of Edwards Lifesciences, Irvine, CA). (c) Self-expanding Medtronic CoreValve® bioprosthesis (Courtesy of Medtronic, Minneapolis, MN)
Self-Expandable Bioprostheses
The present third generation of the CoreValve bioprosthesis (Fig. 27.1c) is constructed of an optimized (maximal radial force, anatomic adaptation), self-expandable nitinol frame with integrated tricuspid porcine pericardial valve. It is released by withdrawing the outer catheter sheath. Contrary to balloon-expandable systems, no tachycardic pacing is required during prosthesis expansion. The valve is available in sizes of 23, 26, 29, and 31 mm for a retrograde procedure, via the femoral/subclavian/axillary artery, or via a direct transaortic access. Whereas the first prosthesis generation required a 25F sheath, the procedure is performed now using an 18F sheath.
Approaches
Supported by the catheter, the valve prosthesis can be brought to the native aortic valve via two different routes: (1) anterograde, i.e., following blood flow, from the left ventricle via a transapical access; or (2) retrograde, i.e., via the ascending aorta applying a transarterial procedure. Transarterial, i.e., the transfemoral procedure or access via A. subclavia or A. axillaris or as an alternative the direct, transaortic procedure have proven valuable.
The transapical approach (TA-AVR; Fig. 27.2) requires a small left lateral minithoracotomy with direct puncture of the left ventricular apex and was first described in 2006 by Litchenstein et al. [19]. Advantages of the TA-AVR are (1) the very good control of the prosthesis during positioning and implantation due to the very short distance between the vascular access and the native aortic valve, and (2) that there is no need for peripheral arterial catheter passage, especially with pre-existing peripheral artery disease. TA-AVR is mainly performed under general anesthesia in the operating room or in a hybrid-OR. Case reports have been published on high epidural anesthesia in TA-AVR, but these are not widely used [20, 21].
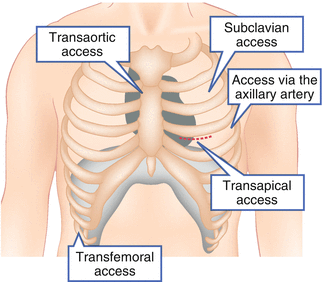

Fig. 27.2
Illustration of the different TAVR approaches
With the continuous reduction of sheath size and improvement in closure devices used, transfemoral TAVR (TF-AVR; Fig. 27.2) has developed nowadays in most centers to the preferred procedure. As a purely percutaneous procedure, TF-AVR is performed under local or general anesthesia primarily in the cardiac catheter laboratory or in the hybrid-OR. The access via the subclavian or axillary artery (TS-AVR; Fig. 27.2) is considered as an alternative to the transfemoral approach [21] and is reported in a proportion of 20 % in an Italian registry. Due to the anatomical site, TS-AVR usually requires surgical exposure of the artery. An advantage of the axillary over the subclavian approach is that vascular injuries can be treated more easily.
Direct, transaortic access through a small right or medial sternotomy (TAO-AVR; Fig. 27.2) was first presented in 2009 as a retrograde alternative [22]. Analogous to the TA- and TS-AVR, the short distance to the aortic valve enables optimal catheter control. Moreover, there is an advantage that no peripheral arterial catheter passage or apical puncture is necessary. However, due to the risk of severe intrathoracic bleeding, TAO-AVR is indicated only for a few selected patients (e.g., if alternative access routes are not feasible).
Clinical Studies and Results
The PARTNER trial and the CoreValve High Risk Study are currently the only prospective, randomized TAVR studies. The most important results are shown in Table 27.3. In the CoreValve High Risk Study, only patients with severe aortic valve stenosis who were at increased risk were included. In PARTNER patients were enrolled who were either at increased operative risk (Cohort A) or inoperable (Cohort B). Cohort A compared TF- and TA-AVR with surgical aortic valve replacement (SAVR) [23, 24]. In Cohort B, TF-AVR was tested in comparison with a standard therapy [13]. Moreover, several multicenter registries have been published in recent years. An overview of the registries with the largest number of patients is found in Table 27.3. It should be noted that the registries consist primarily of inoperable and high-risk patients (EuroSCORE >20 %, STS Score >8 %) [8, 9, 25–29]. Furthermore, most patients present with a mean age above 80 years and with numerous comorbidities, like coronary (>50 %) and peripheral artery disease, as well as chronic kidney and pulmonary disease (>20 %).
Table 27.3
Randomized controlled trials and multicenter registries
Study | n | Approach (n) | Valve type | Euro Score I (%) | Success rate (%) | 30-day mortality (%) | 1-year mortality (%) | Stroke (%) | Major vascular complications (%) | Permanent pacemaker (%) |
|---|---|---|---|---|---|---|---|---|---|---|
Randomized controlled trials | ||||||||||
358 | TF: 179 | SAPIEN® | 26.4 ± 17.2 | 98.8 | 5 | 30.7 | 6.7 | 16.2 | 3.4 | |
PARTNER B [1] (high-risk cohort) | 699 | TF: 244 | SAPIEN® | 29.3 ± 16.5 | N/A | 3.4 (TF: 3.3; TA: 3.8) | 24.2 (TF: 22.2; TA: 29) | 4.6 | 11 | 3.8 |
TA: 104 | ||||||||||
CoreValve High Risk Study | 795 | TF | CoreValve® | 17.6 ± 13.0 | N/A | N/A | 14.2 % vs. 19.1 % | N/A | 5.9 % vs. 1.7 % | 19.8 % vs. 7.1 % |
Registries | ||||||||||
Canadian [24] | 339 | TF: 162 | Cribier-Edwards: 57 | 27.7 ± 16.3 | 93.3 | 10.4 | 24 | 2.3 | 13 | 4.9 |
TF: 90.5 | TF: 9.5 | TF: 25 | TF: 3.0 | TF: 13.1 | TF: 3.6 | |||||
TA: 1.7 | ||||||||||
TA: 13.0 | TA: 6.2 | |||||||||
TA: 11.3 | TA: 22 | |||||||||
SAPIEN®: 275 | TA: 96.1 | |||||||||
TF: 25.8 ± 14.9 | ||||||||||
SAPIEN XT®: 7 | TA: 29.4 ± 17.2 | |||||||||
TA: 177 | ||||||||||
European [19] | 646 | All TF | CoreValve® | TF: 23.1 ± 13.8 | TF: 97.2 | TF: 8.0 | N/A | TF: 1.9 | TF: 1.9 | TF: 9.3 |
German [22] | 697 | TF: 644 | SAPIEN®: 109 | 20.5 ± 13.2 | 98.4 | 12.4 | N/A | 2.8 | 19.5 | 39.3 |
SAPIEN®: 22.0 | ||||||||||
CoreValve®:42.5 | ||||||||||
CoreValve®: 588 | ||||||||||
TS: 22 | ||||||||||
TA: 26 | ||||||||||
TAO: 5 | ||||||||||
Italian [20] | 663 | TF: 599 | CoreValve® | 23.0 ± 13.7 | 98 | 5.4 | 15 | 1.2 | 2 | 16.6 |
TS: 64 | ||||||||||
SOURCE [3] | 1038 | TF: 463 | SAPIEN® | TF: 25.7 ± 14.5 | 93.8 | 8.5 | 23.9 | 2.5 | 7 | 7 |
TA: 575 | TF: 95.2 | TF: 6.3 | TF: 18.9 | TF: 2.4 | TF: 10.6 | TF: 6.7 | ||||
TA: 10.3 | TA: 2.4 | TA: 7.3 | ||||||||
TA: 92.7 | ||||||||||
TA: 27.9 | TA: 2.6 | |||||||||
TA: 29.1 ± 16.3 | ||||||||||
UK [23] | 870 | TF: 599 | SAPIEN®: 410 | N/A | 97.2 | 7.1 | 21.4 | 4.1 | 6.3 | 16.3 |
CoreValve®: 452 | ||||||||||
Others: 271 | ||||||||||
Procedural Success and Hemodynamics
The feasibility of the procedure is clearly demonstrated in clinical practice with a procedural success rate of >90 % and an extremely low conversion rate to SAVR (0.5–2.3 %). The short- and long-term hemodynamic results after TAVR are also convincing, with a mean gradient of <15 mmHg and an orifice area >1.5 cm [2] (Fig. 27.3) [6–9, 16, 23–26, 28–40]. Moreover, hemodynamics after TAVR, analogous to SAVR, result in an improvement of left ventricular function in most patients, especially in patients with LVEF <50 % [41–43]. It is, however, decisive that the current results do not indicate any basic long-term problems for TAVR.
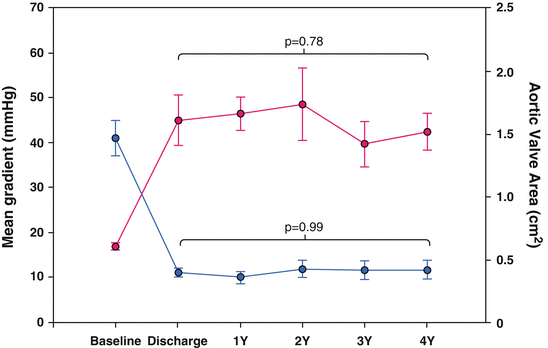

Fig. 27.3
Short- and long-term hemodynamic results after TAVR, with a mean residual gradient of < 15 mmHg and an orifice area >1.5 cm2. Data from Rodes-Cabau J, Webb J, Cheung A, Ye J, Dumont E, Chris F, Osten M, Natarajan M, Velianou JL, Martucci G, DeVarennes B, Thompson CR, Chisholm R, Peterson M, Lichtenstein S, Toggweiler S, Doyle D, DeLarochelli R, Dumesnil J, Teoh K, Chu V, Cheema A, Wood D, Pibarot P, Horlick E. Long-term outcomes following transcatheter aortic valve implantation: Insights on prognostic factors and valve durability from the Canadian multicenter experience. Journal of the American College of Cardiology. 2012;59:E10–E10
NYHA Class and Quality of Life
TAVR improves the patient’s symptoms and NYHA Class, respectively [6–9, 16, 23–26, 28–40]. While most patients have NYHA Class III prior to the procedure, NYHA Class I or II could be attained even in the mid-term course [7, 9, 44]. Improvement in exercise capacity was objectified using the standardized 6-min walking test [44]. Several studies have also shown a positive effect on the quality of life [45–48]. Due to the numerous, sometimes serious secondary comorbidities (cardiomyopathy, mitral valve regurgitation, COPD, stroke, dialysis) and also because of the advanced age, not all patients benefit from TAVR [47, 49]. Future studies will help to identify patients with a positive benefit-risk ratio.
Mortality
PARTNER B (inoperable patients) showed a clear survival advantage for transcatheter aortic valve replacement [7]. The 1-year mortality was 30.7 % vs. 50.7 % in the conservative treatment group (p < 0.01). Patients treated with the SAPIEN THV (exclusively TF-AVR) had an absolute reduction in mortality at 1 year of 20 %, with a number needed to treat (NNT) of 5. PARTNER A, designed to prove the non-inferiority of TAVR compared to SAVR, confirmed this with respect to the 30-day (TAVR: 3.4 % vs. SAVR: 6.5 %; p = 0.07) and 1-year mortality (TAVR: 24.2 % vs. SAVR: 26.8 %; p = 0.44) [23, 24]. Analogous, the CoreValve High Risk study showed that TAVR with a self-expanding THV is associated with a significantly higher rate of survival at 1 year than surgical aortic valve replacement (14.2 % vs. 19.2 %).
The published studies and registry data systematically show a 30-day mortality of <10 % for the transfemoral and <17 % for the transapical approach [8, 9, 25–29, 40, 50, 51]. It can be assumed that the difference between the two approaches is most likely due to the higher risk profile of the patients undergoing the transapical procedure. The mortality after 1 year was 20 % (TF-AVR: 15–25 %), respectively 30 % (TA-AVR: 22–37 %).
To date, there are only few long-term data on TAVR. In the PARTNER A Cohort, Kodali et al. showed a mortality after 2 years of 33.9 % for TAVR and 35 % for SAVR (p = 0.78) [24]. In the 2-year follow-up, the PARTNER B Cohort confirmed the significant survival advantage found after 1 year for TAVR compared to conservative treatment (TF-AVR: 56.7 % vs. Standard therapy: 32.4 %; p < 0.01) [52]. Buellesfeld et al. reported a 2-year survival rate of 72 % for the CoreValve System [53]. A small Edwards collective (88 patients, treated with the Cribier-Edwards or Edwards SAPIEN valves) showed a survival rate of 51 % after 3 years [54]. Comparable results with survival rates of 52 % after 3 years and 50 % after 4 years are found in the Canadian Registry [55]. When one considers the learning curve effect and the technical advances, further improvement can be expected in the future [56, 57].
Complications
In assessing TAVR complications, it must be remembered that a standardized consensus definition of the relevant clinical endpoints and complications was not made by the Valve Academic Research Consortium (VARC) until 2011 [58]. Differences in the published complication rates may thus sometimes be attributed to this situation. In addition, the continuous technical advances (such as smaller catheter systems, prosthesis design) and the learning curve effect may explain discrepancies encountered over time. The most important complications associated with TAVR are listed in Table 27.3.
Cerebrovascular Events
A decisive factor for the acceptance and future expansion of the indication spectrum for TAVR is certainly the frequency of cerebrovascular events. A meta-analysis of 10,037 published cases showed an event rate (TIA and stroke) of 3.3 % in the first 30 days after TAVR [59]. In the majority of cases, serious stroke occurred. These were accompanied by a 3.5-fold higher mortality. MRI studies also show postinterventional “silent” cerebral insults in 66–84 % of TAVR patients, occurring independently of valve type and route of access [60–62]. Decisive, predisposing factors for the occurrence of stroke are a newly occurring atrial fibrillation and a higher-grade mitral valve insufficiency [63]. Moreover, the antithrombotic and anticoagulatory regime appear to play a major role. There are presently no clear recommendations from the specialist societies in this respect.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








