Neurologic Complications of Peripheral Nerve Blocks
Jeff Gadsden
Nerve injury following peripheral nerve blockade (PNB) is a potentially devastating complication that can result in permanent disability.1 Data from a recent review of published studies suggest that the incidence of neurologic symptoms following PNB varies depending on the anatomic location, ranging from 0.03% for supraclavicular blocks to 0.3% for femoral blocks to up to 3% for interscalene blocks.2 Fortunately, the vast majority of these neuropathies appear to be temporary rather than permanent neuropathy and resolve over weeks to months.
The exact etiology of neurologic injury related to PNB remains unclear in many instances. Suggested etiologies include mechanical trauma from the needle, nerve edema and/or hematoma, pressure effects of the local anesthetic injectate, and neurotoxicity of the injected solutions (both local anesthetics and adjuvants, e.g., epinephrine).3 Confounding factors that may play a role in nerve injury include preexisting neuropathies (e.g., diabetes mellitus), surgical manipulation, prolonged tourniquet pressure, or compression from postoperative casting.4
It is well-established that direct injection into peripheral nerves (i.e., accidentally during intramuscular administration) can result in nerve injury.5 This is one of the reasons why intraneural injections are avoided during peripheral nerve blockade. More recent data however, suggest that intraneural injections are not always associated with nerve injury. This chapter summarizes the clinically relevant considerations regarding the etiology of nerve injury during peripheral nerve blockade.
Histology and Histopathology of Peripheral Nerves
Knowledge of the functional histology of nerves is essential to understanding the consequences of intraneural injection. Nerves are made up of fascicles supported and enveloped by perineurium and a loose collection of collagen fibers termed the epineurium. The epineurium is easy permeable and carries the nutritive vessels of larger nerves. Each fascicle is made up of bundles of nerve fibers (axons) and their associated Schwann cells held together by a tough squamous epithelial sheath called the perineurium, which acts as a semipermeable barrier to local anesthetics. The nerve fibers are supported within the perineurium by a delicate connective tissue matrix called the endoneurium, which contains capillaries that arise from the larger epineurial vessels. Figure 10-1 features normal anatomy of a mixed peripheral nerve and relationship of the epineurium and perineurium.
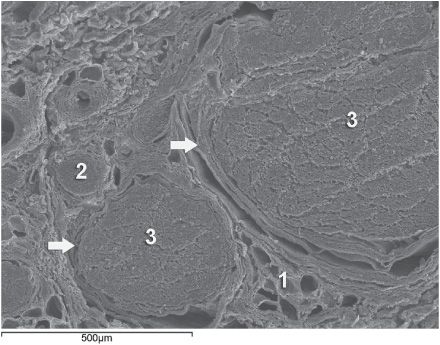
FIGURE 10-1. Anatomy of the peripheral nerve as seen on an electron microscopy image. 1-Epineurium, 2-Fascicle, 3-Fascicular bundles (several fascicles bound together), Arrows-perineurium.
Peripheral nerve lesions can be classified in terms of their degree of functional disruption.6 Neurapraxia refers to a mild insult in which the axons and connective tissue structures supporting them remain intact. This type of injury is often associated with focal demyelination and generally reversible over the course of weeks to several months. Axonal interruption with conservation of the neural connective tissues is termed axonotmesis. Regeneration at a rate of 1 to 2 mm/day occurs, and recovery is generally favorable although not always complete. Neurotmesis represents complete fascicular interruption, including the axons and the connective tissue supporting tissues. Because the nerve is severed, recovery depends on surgical reapproximation of the two stumps. Even with prompt surgical intervention, recovery is often poor. It is important to note that most nerve injuries are mixed, with different fascicles exhibiting characteristics of these three different injury types.
The Problem, or Is It?
Selander et al provided evidence of the deleterious effects of intraneural injection over 30 years ago7 Indeed, the objective during peripheral nerve blockade has been to deposit local anesthetic in the vicinity, but not within, the substance of the nerve. This tacit convention has been challenged in recent years with the publication of a series of reports suggesting that intraneural needle placement, and indeed injection of local anesthetic, may not necessarily result in detectable clinical injury. In 2004 Sala-Blanch et al described two cases of placement of a catheter within the epineurium of the sciatic nerve, confirmed by computerized tomographic imaging.8 Both patients demonstrated clinically efficacious blocks without postoperative neurologic deficit. The advent of ultrasound guidance for nerve blocks has likely led to an increase in the recognition of inadvertent intraneural injections. Accidental femoral9 and musculocutaneous10 intraneural injections have been described, as evidenced by nerve swelling on the ultrasound image, both without lasting neurologic effect.
In 2006, Bigeleisen published a series of axillary brachial plexus blocks performed on 22 patients undergoing thumb surgery.11 Using ultrasound guidance, the authors deliberately placed the needle intraneurally and injected 2 to 3 mL of local anesthetic, which resulted in 72 intraneural injections as evidenced by nerve swelling. Despite the common occurrence of paresthesia or dysesthesia (66 times), none of the patients developed an overt neurologic deficit up to 6 months postoperatively.
Similarly, Robards et al studied 24 patients receiving sciatic nerve blocks in the popliteal fossa using both nerve stimulation and ultrasound guidance.12 The end point for needle advancement was a motor response using a current intensity of 0.2 to 0.5 mA, or an apparent intraneural needle tip location, whichever came first. These investigators found that the motor response could only be obtained upon entry of the needle into the nerve in 83.3% of patients; in the remaining 16.7%, a motor response with a stimulating current of 1.5 mA could not be obtained, even when the needle tip was intraneural. There was no postoperative neurologic dysfunction.
Taken together, these studies suggest that an intraneural needle placement with resultant injection of the local anesthetic within internal epineurium does not lead to an imminent neurologic injury. The data by Robards et al, suggest that many nerve blocks without the benefit of ultrasound visualization, have likely resulted in intraneural (intra-epineural) injections. The reason why nerve injury is infrequent is that the vast majority of these injections do not occur within fascicles.
Extrafascicular versus Intrafascicular Injections
A needle placed within a peripheral nerve can be in one of two locations: within the loose epineurial matrix that surrounds the fascicles or inside a fascicle itself. It is well-established that injection of even very small amounts of local anesthetic within the fascicle can lead to widespread axonal degeneration and permanent neural damage in animals, whereas extrafascicular injection does not disrupt the normal nerve architecture.7,13 Part of this can be explained mechanically because the perineurium, a tough multilayer epithelial sheath, is not easily distensible to compensate to an increase in intrafascicular pressure. Intrafascicular pressure rises on injection and can remain higher than the capillary perfusion pressure longer than the duration of the injection itself, predisposing to neural ischemia and inflammation.14 Furthermore, pressure curves derived from intrafascicular versus extrafascicular injections in canine sciatic nerves show that a pattern of very high initial injection pressures followed by a sharp drop to baseline is associated with poor outcome and severe neural histologic damage, and may suggest fascicular rupture.15 In contrast, injections into the compliant epineurial space appear to be associated with a minimal rise in pressure, which can be explained by its loose and accommodating stromal architecture.
The risk of an intrafascicular injection differs from site to site in the peripheral nervous system, and it correlates with the cross-sectional fascicle-epineurium ratio. For example, the sciatic nerve at the popliteal fossa contains more nonneural tissue than fascicles in its cross-sectional area, which corresponds with its low incidence of post-PNB neuropathy.16 By contrast, the brachial plexus at the level of the trunks contains much more neural than connective tissue; a needle entering the nerve here is more likely to encounter a fascicle on its trajectory that may contribute to the disproportionately higher rate of postoperative neuropathy following PNB with interscalene blocks.17 As peripheral nerves move away from the neuraxis, the ratio of connective tissue to neural tissue within the nerve tends to increase. The brachial plexus elements below the clavicle have a ratio of connective tissue to neural tissue of approximately 2:1, whereas the more proximal trunks and divisions have a ratio of 1:1.18
Mechanisms of Nerve Injury Following Intraneural Injection
Once the perineurium is breached, the spectrum of subsequent injury is wide and multifactorial.
Needle Trauma
The mechanical disruption of the perineurial sheath may result in injury to the axons and/or the leakage/herniation of endoneural contents.19 However, the composition of the injectate may play a larger role in the outcome of intrafascicular injection. For example, normal saline injected into fascicles did not cause any damage in one study, suggesting that mere puncture of the perineurium does not necessarily result in clinically overt injury.13 In contrast, nerve puncture with intravenous cannulae or electroneurography needles has been shown to result in lasting neurologic deficit.20–22 A variety of cellular changes accompany needle trauma, including alterations in membrane channel expression, activation of signal transduction, neuropeptide production, and an overall increase in excitability at the dorsal horn.23,24 The effect of the needle size on the likelihood and severity of the injury is controversial, however, smaller needles (24 gauge) may lead to less nerve injury than larger needles (19 gauge).25
Despite the concern over fascicular puncture, due to their compact nature, fascicles are more likely to escape the advancing needle, rather than be penetrated under normal PNB conditions. Early work by Selander et al in rabbits demonstrated that needle tip characteristics influenced the likelihood of fascicular penetration.26 This study demonstrated that long-bevel (12–15°) needles were more likely to puncture the fascicle than short-bevel (45°) needles, and resulted in the author advocating for their use during PNB. A more recent study compared blunt (30°) versus sharp (15°) needles by passing these needles through a cadaveric sciatic nerve and examining the nerve microscopically afterward for signs of fascicular damage.27 Although a total of 134 fascicles were identified as being in contact with the needle tracks, only 4 fascicles were damaged, all of which belonged to the sharp-tip group. These data suggest that a needle passing through a fascicle is more likely only to encounter epineurium and may in fact displace the tough fascicles away from the needle path. Although blunt needles are less likely to enter the fascicle, once penetrated, blunt needles appear to cause a greater degree of injury compared with sharp needles, especially if the sharp needles are oriented with the bevel in the same direction as the nerve fibers (i.e., not cutting transversely across the fibers).28 Regardless of which needle type or size enters the nerve, a needle insertion into nerve and consequent injection invariably leads to inflammation and cellular infiltration regardless of whether a clinical injury occurs.
Toxicity of Local Anesthetics and Additives
Although all local anesthetics are potentially neurotoxic,29 the mechanism remains unclear. Proposed mechanisms include increases in intracellular calcium concentration, disturbance in mitochondrial function, interference with membrane phospholipids, and cell apoptosis.30–33 The perineurium and blood vessel endothelium serve as a barrier to entry into the fascicle. However, even local anesthetics placed within the epineurium have been shown to cause altered perineural permeability and fascicular edema, leading to compression of the fascicle and reduced neural blood flow.13,34 This effect appears to be dose dependent.
Intraneural administration of local anesthetics exposes the axons to higher concentrations of drug than extraneural application. One study comparing the extraneural, extrafascicular, and intrafascicular administration of ropivacaine 0.75% showed that histologic damage was least severe extraneurally and most severe intrafascicularly.35 However, even when injected inside the epineurium, others have shown ropivacaine 0.75% to have no adverse effect on functional recovery.36 Ester local anesthetics such as tetracaine and chloroprocaine were shown in some studies to cause a greater degree of injury than those of the amide group, but recent data have challenged those conclusions.34,37 What is well known is that the injection of local anesthetics into the fascicle results in widespread and immediate axonal injury.14
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








