HISTORY AND CURRENT RELEVANCE
As one of the earliest recognized complications of regional anesthesia, MPH has a long and colorful history.6 Dr. August Bier noted this adverse effect in the first patient to undergo successful spinal anesthesia on August 16, 1898. Bier7 observed: “Two hours after the operation his back and left leg became painful and the patient vomited and complained of severe headache. The pain and vomiting soon ceased, but headache was still present the next day” (italics added). The following week, Bier and his assistant, Dr. August Hildebrandt, performed experiments with cocainization of the spinal cord on themselves. In a description of MPH scarcely improved upon in an intervening century, Bier7 later reported firsthand his experience in the days to follow: “I had a feeling of very strong pressure on my skull and became rather dizzy when I stood up rapidly from my chair. All these symptoms vanished at once when I lay down flat, but returned when I stood up … I was forced to take to bed and remained there for nine days, because all the manifestations recurred as soon as I got up …. The symptoms finally resolved nine days after the lumbar puncture.” In medical history, few complications have come to be considered as closely linked to a specific technique as MPH with spinal anesthesia.
Employing the methods of the early 20th century, spinal anesthesia was frequently followed by severe and prolonged headache, casting a long shadow over the development and acceptance of this modality. Investigations into the cause of these troubling symptoms eventually led to the conclusion that they were due to persistent cerebrospinal fluid (CSF) loss through the rent created in the meninges. The most notable successful efforts to minimize the loss of CSF were through the use of smaller gauge and “noncutting” needles (as convincingly demonstrated in the 1950s by Vandam and Dripps8 and Hart and Whitacre,9 respectively). Despite these significant advances in prevention, MPH remained a frustratingly common occurrence.
The extensive search for effective treatments for MPH dates to Bier’s time. Yet efforts through the first half of the 20th century, while often intensive and creative, were questionably worthwhile. In a monograph intended to be a comprehensive review of MPH from the 1890s through 1960, Dr. Wallace Tourette et al.10 cite dozens of separate and far-ranging treatment recommendations, including such interventions as intravenous ethanol, x-rays to the skull, sympathetic blocks, and manipulation of the spine. Unfortunately, prior to the introduction of the EBP, there were no treatment measures that could be described as significant improvements over the simple passage of time. In his 1955 textbook, Complications of Regional Anesthesia, Dr. Daniel C. Moore11 describes in detail a full 3-day treatment protocol for MPH. He concludes by noting that 3 days is the usual duration of untreated mild-to-moderate headaches, but that “nevertheless, the patient feels an attempt to help his problem is being made.”
The EBP, a startlingly unique medical procedure, proved to be the major breakthrough in the treatment of MPH. The concept of using autologous blood to “patch” a hole in the meninges was introduced in late 1960 by Dr. James Gormley,12 a general surgeon. Yet, Gormley’s brief report went largely unnoticed for nearly a decade because, to the practitioners of the day, an iatrogenic epidural hematoma raised serious concerns of scarring, infection, and nerve damage. The procedure was only later popularized in anesthesiology circles, and performed as a true epidural injection, largely through the work of Drs. Anthony DiGiovanni and Burdett Dunbar.13 The EBP procedure was further refined through the 1970s as the volume of blood commonly utilized increased to 20 mL.14 Today, the EBP is nearly universally employed as the cornerstone of treatment for severe MPH.15
MPH remains a prominent clinical concern to the present day. Largely due to modifications in practice that followed the identification of risk factors, rates of MPH following spinal anesthesia have steadily declined—from an incidence exceeding 50% in Bier’s time to around 10% in the 1950s,8 until currently a rate of 1% or less can be reasonably expected. However, as perhaps the highest risk group, an unfortunate 1.7% of obstetric patients continue to experience MPH after spinal anesthesia using 27-gauge Whitacre needles.16 Intending to avoid meningeal puncture, epidural techniques are an attractive alternative to spinal anesthesia. Yet occasional UDP, either with the needle or the catheter, is unavoidable (and may be unrecognized at the time in over 25% of patients who eventually develop MPH17). In nonobstetric situations (e.g., interlaminar epidural steroid injections), the rate of UDP should be <0.5%. However, UDP is of greatest concern in the obstetric anesthesia setting, where the incidence of this adverse event is around 1.5%.16 Over half of all patients who experience UDP with epidural needles will eventually develop headache symptoms, with many studies in obstetric populations reporting MPH rates of 75% or greater. In addition to anesthesia interventions, MPH remains a too-common iatrogenic complication following myelography and diagnostic/therapeutic lumbar puncture (LP). In these situations, rates of MPH around 10% are still commonly cited as practitioners often continue to use Quincke needles, and large-gauge needles are considered necessary due to the viscosity of contrast material and to facilitate the timely collection of CSF. Consequently, there is evidence to suggest that the majority of instances of MPH now have a non–anesthesia-related origin.18
The practical significance of MPH is illustrated in being noted in the American Society of Anesthesiologists Closed Claims Project database as one of the most frequent claims for malpractice involving obstetric anesthesia,19 regional anesthesia,20 and chronic pain management.21 Justifiably, headache is the most commonly disclosed risk when obtaining consent for spinal and epidural anesthesia.22 The potentially serious nature of this complication necessitates inclusion in informed consent involving any procedure that may result in MPH. As part of this discussion, patients should also be apprised of the normal delayed onset of symptoms and given clear instructions for the timely provision of advice or management should they experience adverse effects.
 PATHOPHYSIOLOGY
PATHOPHYSIOLOGY
It has long been accepted that MPH results from a disruption of normal CSF homeostasis. However, despite a great deal of research and observational data, the pathophysiology of MPH remains incompletely understood.23
CSF is produced primarily in the choroid plexus at a rate of approximately 0.35 mL/min and reabsorbed through the arachnoid villa. The total CSF volume in adults is maintained around 150 mL, of which approximately half is extracranial, and gives rise to normal lumbar opening pressures of 5 to 15 cm H2O in the horizontal position (40–50 cm H2O in the upright position). It has been shown experimentally that the loss of approximately 10% of total CSF volume predictably results in the development of typical MPH symptoms, which resolve promptly with the reconstitution of this deficit.24 It is agreed that MPH is due to the loss of CSF through a persistent leak in the meninges. In this regard, it has been postulated that the cellular arachnoid mater (containing frequent tight junctions and occluding junctions) is perhaps more important than the more permeable and acellular dura mater in the genesis of symptoms.3 Thus, preference for the term “meningeal puncture headache” over “postdural puncture headache”. The apparent role of the arachnoid mater in this disorder further calls into question the significance of many published studies that involve isolated dura mater in vitro.
The actual means by which CSF hypotension generates headache is controversial and currently ascribed to a bimodal mechanism involving both loss of intracranial support and cerebral vasodilation (predominantly venous). Diminished buoyant support is thought to allow the brain to sag in the upright position, resulting in traction and pressure on pain-sensitive structures within the cranium (dura, cranial nerves, bridging veins, and venous sinuses) (Fig. 9-1). Passive and adenosine-mediated vasodilation may occur secondary to diminished intracranial CSF (in accordance with the Monro-Kellie hypothesis, which states that intracranial volume must remain constant) and reflexively secondary to traction on intracranial vessels.
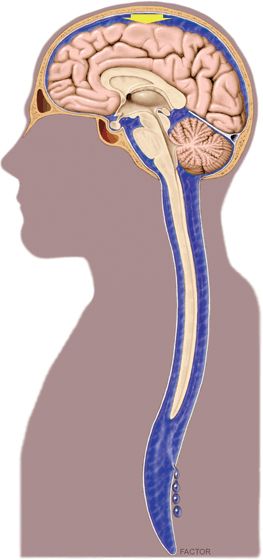
FIGURE 9-1. Schematic drawing illustrating diminished buoyant support of intracranial structures in the upright position (arrow) secondary to persistent extracranial CSF loss. (Image by David Factor [Mayo Clinic, Rochester, MN], with his permission.)
Multiple neural pathways are involved in generating the symptoms of MPH. These include the ophthalmic branch of the trigeminal nerve (CN V1) in frontal head pain, cranial nerves IX and X in occipital pain, and cervical nerves C1-3 in neck and shoulder pain.25 Nausea is attributed to vagal stimulation (CN X). Auditory and vestibular symptoms are secondary to the direct communication between the CSF and the perilymph via the cochlear aqueduct, which results in decreased perilymphatic pressures in the inner ear and an imbalance between the endolymph and perilymph.26 Significant visual disturbances may represent a transient palsy of the nerves supplying the extraocular muscles of the eye (CN III, IV, and VI). Here, the lateral rectus muscle is most often involved, which is attributed to the long, vulnerable intracranial course of the abducens nerve (CN VI).27 Other, much less frequent cranial nerve palsies of the trigeminal (CN V), facial (CN VII), and auditory (CN VIII) nerves have also been reported.28
 CLINICAL PRESENTATION AND CHARACTERISTICS
CLINICAL PRESENTATION AND CHARACTERISTICS
Although many clinical variations have been described, most cases of MPH are characterized by their typical onset, presentation, and associated symptoms.
Onset
Onset of symptoms is generally delayed, with headache usually beginning 12 to 48 hours and rarely more than 5 days following meningeal puncture. In their landmark observational study, Vandam and Dripps8 reported onset of headache symptoms within 3 days of spinal anesthesia in 84.8% of patients for whom such data were available. More recently, Lybecker et al.29 performed a detailed analysis of 75 consecutive patients with MPH following spinal anesthesia (primarily using 25-gauge cutting-point needles). While none of their patients noted the onset of symptoms during the first hour following meningeal puncture, 65% experienced symptoms within 24 hours and 92% within 48 hours. An onset of symptoms within 1 hour of neuraxial procedures is suspicious for pneumocephalus, especially in the setting of an epidural loss-of-resistance technique using air.30 Occasional reports of unusually delayed onset of MPH highlight the importance of seeking a history of central neuraxial instrumentation whenever positional headaches are evaluated.31
Presentation
The cardinal feature of MPH is its postural nature, with headache symptoms worsening in the upright position and relieved, or at least improved, with recumbency. The International Classification of Headache Disorders further describes this positional quality as worsening within 15 minutes of sitting or standing and improving within 15 minutes after lying.1 Headache is always bilateral, with a distribution that is frontal (25%), occipital (27%), or both (45%).29 Headaches are typically described as “dull/aching,” “throbbing,” or “pressure-type.”
The severity of headache symptoms, a feature with important ramifications for treatment, varies considerably among patients. Although there is no widely accepted severity scale, one practical approach is to have patients simply rate their headache intensity using a 10-point analog scale, with 1 to 3 classified as “mild,”, 4 to 6 “moderate,” and 7 to 10 “severe.” Lybecker et al.29 further categorized patients according to restriction in physical activity, degree of confinement to bed, and presence of associated symptoms (Box 9-1). A prospective analysis of MPH after spinal anesthesia using Lybecker’s classification system demonstrated that 11% were mild, 23% moderate, and 67% severe.
BOX 9-1 Classification of Severity of MPH
Severity of MPH

Associated Symptoms of MPH

Adapted from Lybecker H, Djernes M, Schmidt JF. Postdural puncture headache (PDPH): onset, duration, severity, and associated symptoms. An analysis of 75 consecutive patients with PDPH. Acta Anaesthesiol Scand 1995;39:606–12, with permission.
Associated Symptoms
If headaches are severe, they are more likely to be accompanied by a variety of other symptoms. Pain and stiffness in the neck and shoulders is common and seen in nearly half of all patients experiencing MPH.32 With questioning, nausea may be reported by a majority of patients and can lead to vomiting.29
Uncommonly, patients may experience auditory or visual symptoms,26 and the risk for either appears to be directly related to needle size.27,33 In Vandam and Dripps’8 large study of MPH, each was seen to a clinically apparent degree in 0.4% of patients. Auditory symptoms include hearing loss, tinnitus, and even hyperacusis and can be unilateral. It is interesting to note that subclinical hearing loss, especially in the lower frequencies, has been found to be common following spinal anesthesia, even in the absence of MPH.33 Closely associated with auditory function, vestibular disturbances (dizziness or vertigo) may also occur. Visual problems include blurred vision, difficulties with accommodation, mild photophobia, and diplopia. In contrast to headache complaints, which are consistently bilateral, nearly 80% of episodes of diplopia secondary to meningeal puncture involve unilateral cranial nerve palsies.27
 RISK FACTORS
RISK FACTORS
Risk factors for MPH can be broadly categorized into patient characteristics and procedural details.
Patient Characteristics
The patient characteristic having the greatest impact on risk of MPH is age. Uncommonly reported in children <10 years of age, MPH has a peak incidence in the teens and early 20s.34 The incidence then declines over time, becoming much less frequent in patients over 50 years of age (Fig. 9-2). Females have long been recognized as being at increased risk for MPH, and a systematic review of published studies found the odds of developing MPH were significantly lower for male than age-matched nonpregnant female subjects (odds ratio = 0.55; 95% confidence interval, 0.44–0.67).35 The etiology behind this gender difference is not clear. Body mass index (BMI) appears to be a mixed risk factor. Morbid obesity presents obvious technical difficulties for central neuraxial procedures, increasing the likelihood of multiple needle passes and UDP.36 Yet, low BMI has been reported as an independent risk factor for MPH37 and high BMI (i.e., obesity) may actually decrease the risk, possibly due to a beneficial effect of increased intra-abdominal pressure.38
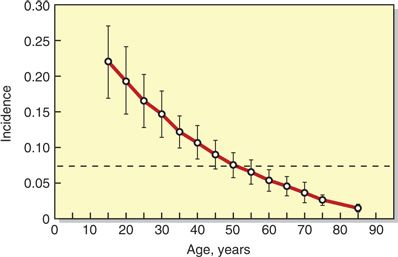
FIGURE 9-2. Logistic regression of the incidence of MPH as a function of age: Pa = [1 + exp (0.633 + 0.039 × age)]–1. Age-specific incidence means and 95% confidence limits are shown as vertical lines. The overall incidence of MPH for this study, which used cutting needles only, was 7.3% (dashed horizontal line). (Redrawn and used from Lybecker H, Moller JT, May O, et al. Incidence and prediction of postdural puncture headache: a prospective study of 1021 spinal anesthesias. Anesth Analg 1990;70:389–394, with permission.)
Pregnancy has traditionally been regarded as a risk factor for MPH,8 but this consideration largely reflects a young female cohort as well as the high incidence of UDP in the gravid population. Although controversial, pushing during the second stage of labor, thought to promote the loss of CSF through a hole in the meninges, has been reported to influence the risk of MPH following UDP. Angle et al.39 noted that the cumulative duration of bearing down correlated with the risk of developing MPH in patients who had experienced UDP. They also found that patients who avoided pushing altogether (proceeded to cesarean delivery prior to reaching second-stage labor) had a much lower incidence of MPH (10%) than those who pushed (74%).
MPHs appear to have an interesting association with other headaches. Patients who report having had a headache within the week prior to LP have been observed to have a higher incidence of MPH.37 Upon further analysis, only those with chronic bilateral tension-type headaches were found to be at increased risk.40 A history of unilateral headache40 or migraine41 has not been linked to an increased risk of MPH. Menstrual cycle, a factor in migraine headaches, did not influence the rate of MPH in one small pilot study.42 Patients with a history of previous MPH, particularly women, appear to have an increased risk for new MPH after spinal anesthesia.43 With epidural procedures, patients with a history of UDP have been shown to be at slightly increased risk for another UDP (and subsequent MPH).44
Procedural Details
Needle size and tip design are the most important procedural factors related to MPH45 (Fig. 9-3). Needle size is directly related to the risk of MPH. Meningeal puncture with larger needles is associated with a higher incidence of MPH,8 more severe headache and associated symptoms,45 a longer duration of symptoms,46 and a greater need for definitive treatment measures.47 Needle tip design is also a major influence, with “noncutting” needles clearly associated with a reduced incidence of MPH when compared with “cutting” (usually Quincke) needles of the same gauge. In general, noncutting needles have an opening set back from a tapered (“pencil-point”) tip and include the Whitacre, Sprotte, European, Pencan, and Gertie Marx needles. Adding to this somewhat confusing terminology, noncutting needles are sometimes still incorrectly referred to as “atraumatic” needles, this despite being shown with electron microscopy to produce a more traumatic rent in the dura than cutting needles (perhaps resulting in a better inflammatory healing response).48 The influence of needle size on the risk of MPH appears to be greatest for cutting needles (e.g., the reduction seen in the incidence of MPH between 22- and 26-gauge sizes is greater for cutting than noncutting needles). Insertion of cutting needles with the bevel parallel to the long axis of the spine significantly reduces the incidence of MPH.49 This observation was for many years attributed to a spreading rather than the cutting of longitudinal-oriented dural fibers. However, scanning electron microscopy reveals the dura to be made of many layers of concentrically directed fibers,50 and the importance of needle bevel insertion is now thought to be due to longitudinal tension on the meninges, particularly in the upright position, and its influence on CSF leakage through holes having differing orientations.
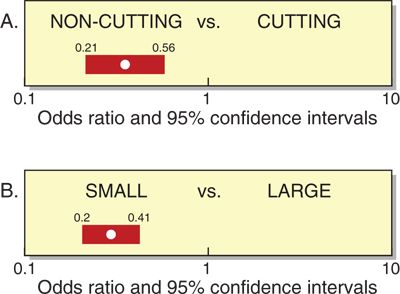
FIGURE 9-3. Pooled odds ratios and 95% confidence intervals (from meta-analysis of nonheterogeneous studies) for risk of MPH based on (A) needle type and (B) needle size. (Redrawn and used from Halpern S, Preston R. Postdural puncture headache and spinal needle design. Metaanalysis. Anesthesiology 1994;81:1376–1383, with permission.)
Not surprisingly, a larger number of meningeal punctures have been shown to increase the rate of MPH.51 The degree of experience/comfort/skill of the operator is clearly associated with the incidence of UDP during epidural procedures, with higher UDP rates consistently reported when procedures are performed by residents.52,53 The risk of UDP also appears to be higher for procedures done at night, strongly suggesting a significant contribution of operator fatigue.54
A number of procedural details do not appear to influence the rate of development of MPH, including patient position at the time of meningeal puncture, “bloody tap” during spinal anesthesia, addition of opiates to spinal block, and volume of CSF removed (for diagnostic purposes).6
 PREVENTION
PREVENTION
Although prophylaxis is most simply thought of as preventing any symptoms of MPH, in the clinical context this issue is deceptively complex. It is important to appreciate that significant “prevention” may encompass a number of other endpoints, such as a reduced incidence of severe MPH, a shorter duration of symptoms, or decreased need for EBP. Unfortunately, despite the clear relevance of this issue, the overall quality of evidence for preventive measures is generally weak.55–57
General Measures
As with all regional techniques, appropriate patient selection is crucial in minimizing complications. In this regard, anesthesiologists should take pause when caring for patients having known risk factors for MPH. As age is a major risk factor, spinal anesthesia is perhaps best avoided in patients under 40 years of age unless the benefits are sufficiently compelling (such as in the obstetric population). Practitioners (and patients alike) may also wish to avoid central neuraxial techniques in those with a previous history of UDP or MPH (particularly females). Other patient-related factors (e.g., obesity) should be considered on a case-by-case basis, weighing the risks of MPH with the benefits of regional anesthesia.
Neuraxial procedures should be performed with needles having the smallest gauge possible. However, extremely small spinal needles are more difficult to place, have a slow return of CSF, may be associated with multiple punctures of the meninges, and can result in a higher rate of unsuccessful block. The ideal choice for spinal anesthesia is generally a 24- to 27-gauge noncutting needle. Epidural options are limited, especially with catheter techniques, but the risk of MPH following UDP can probably also be reduced by always using the smallest feasible epidural needles.
While only recently utilized for neuraxial techniques, the use of ultrasound for regional anesthesia holds some promise in reducing the risk of MPH. Ultrasound can decrease the number of needle passes required for regional procedures and has been shown to accurately predict the depth of the epidural space.58 Further study is ongoing to define this potential for ultrasound to reduce the incidence of UDP and MPH.
Pharmacologic measures, notably caffeine, continue to be widely used in hopes of decreasing the incidence of MPH following meningeal puncture.15 In support of this practice, one small study (n = 60) found that intravenous caffeine (500 mg caffeine sodium benzoate within 90 minutes after spinal anesthesia) significantly reduced the incidence of moderate-to-severe headache.59 However, generalizing these results to other clinical settings is difficult as this investigation involved the use of 22-gauge Quincke needles in a relatively young patient population. In another study, oral caffeine (75 or 125 mg) administered every 6 hours during the first 3 days following spinal anesthesia failed to influence the rate of MPH.60 A critical review of the available evidence fails to support the use of caffeine in the prevention of MPH.61 More recently, a small pilot study raised the possibility of using the long-acting 5-HT receptor agonist frovatriptan (2.5 mg/d orally for 5 days) in the prevention of MPH.62 Currently, however, there is no proven pharmacologic prophylaxis for MPH.
A recent survey of United States (US) anesthesiologists reported that bed rest and aggressive oral and intravenous hydration continue to be employed by a sizable majority as prophylactic measures against MPH.15 However, a systematic review of the literature regarding bed rest versus early mobilization after dural puncture failed to show any evidence of benefit from bed rest and suggested that the risk of MPH may actually be decreased by early mobilization.63 It is notable that the practice of US anesthesiologists regarding bed rest is in direct contrast to that seen in United Kingdom (UK) maternity units, where 75% encourage mobilization as early as possible following UDP as prophylaxis against MPH.64 Likewise, a randomized prospective trial of increased oral hydration following LP failed to decrease the incidence or duration of MPH.63 In summary, at this time there is no evidence to support the common recommendations of bed rest or aggressive hydration in the prevention of MPH.
Spinal Technique
Attention to needle tip design is an important technical means of reducing the risk of MPH with spinal anesthesia. If available, noncutting needles should be employed. If cutting-tip needles are used, the bevel should be directed parallel to the long axis of the spine.
Replacing the stylet after CSF collection but prior to needle withdrawal is an effective means of lowering the incidence of MPH after LP. This recommendation is based on a prospective, randomized study of 600 patients using 21-gauge Sprotte needles. In this setting, replacing the stylet reduced the incidence of MPH from 16.3% to 5.0% (p < 0.005).65 This safe and simple maneuver is theorized to decrease the possibility of a wicking strand of arachnoid mater from extending across the dura (Fig. 9-4).
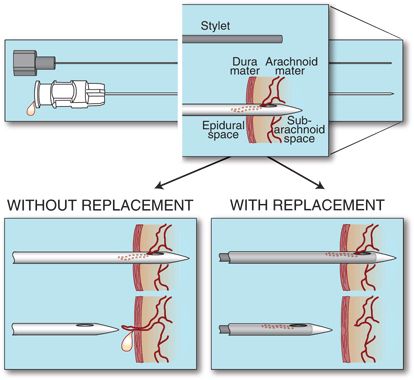
FIGURE 9-4. Schematic drawing of the proposed mechanism of decreased incidence of MPH seen with stylet replacement. Upper: Flow of CSF from the subarachnoid space may draw strands of arachnoid mater into the needle. Lower left: Removal of the needle without stylet replacement results in threading of arachnoid across the dura, promoting prolonged CSF leak. Lower right: Replacing the stylet fully prior to needle removal either pushes out or cuts the arachnoid mater, reducing the risk of CSF loss into the epidural space. (Used from Strupp M, Brandt T, Muller A. Incidence of post-lumbar puncture syndrome reduced by reinserting the stylet: a randomized prospective study of 600 patients. J Neurol 1998;245:589–592, with permission.)
Continuous spinal anesthesia (CSA) has been reported by some to be associated with surprisingly low incidences of MPH compared with single-dose spinal techniques using similar gauge needles.66 This observation has been attributed to the reaction to the catheter, which may promote better sealing of a breach in the meninges. CSA with small-gauge needles and catheters (“microcatheters”) is an appealing option when titration of the spinal drug is desirable and duration of surgery is uncertain, but microcatheters have usually been unavailable in the US, where the risk of MPH with CSA remains a concern when using approximately 20-gauge “macrocatheters.” For this reason, although the technique may have clinical advantages, deliberate CSA has been investigated almost exclusively in low-risk populations.
Epidural Technique
The issue of air versus liquid for identification of the epidural space with the loss-of-resistance technique has long been a source of controversy. Each method has acknowledged advantages and disadvantages but neither has been shown convincingly to result in a lower risk of UDP.67 In this case, operator preference and experience would be expected to strongly influence performance, and the overriding significance of this factor is illustrated in fewer instances of UDP noted when the medium is chosen at the anesthesiologist’s discretion.68
Bevel orientation for epidural needle insertion remains a matter of debate. Norris et al.69 found that the incidence of moderate-to-severe MPH after UDP was only 24% when the needle bevel was oriented parallel to the long axis of the spine (compared to 70% with perpendicular insertion). This resulted in fewer therapeutic EBPs administered to patients in the parallel group (p < 0.05). However, this technique necessitates a controversial 90-degree rotation of the needle for catheter placement.70 It appears that a number of concerns regarding parallel needle bevel insertion (lateral needle deviation, difficulties with catheter insertion, and dural trauma with needle rotation) are of greater concern to practitioners. Most respondents (71.3%) to a survey of US anesthesiologists preferred to insert epidural needles with the bevel perpendicular to the long axis of the spine (consistent with the intended direction of catheter travel).15
Combined spinal-epidural (CSE) techniques have been reported to be associated with a low incidence of MPH. While providing the advantages of a spinal anesthetic, CSE appears to have no increased incidence of MPH or the need for EBP when compared to plain epidural analgesia.71 This observation may be due to several factors, including the ability to successfully use extremely small (e.g., 27-gauge) noncutting spinal needles, and tamponade provided by epidural infusions.
Measures to Reduce the Risk of MPH after UDP
The risk-to-benefit ratio of prophylaxis should be most favorable in situations having the greatest likelihood of developing severe MPH. Therefore, most efforts to reduce the risk of MPH after UDP have been in the obstetric patient population. Several prophylactic measures, discussed below, are worthy of consideration and have been utilized alone or in combination.72 However, since not all patients who experience UDP will develop MPH, and only a portion of those who do will require definitive treatment (i.e., an EBP), a cautious approach in this regard is still generally warranted.
Stylet Replacement
Although there have not been any studies to support the use of this technique in the setting of UDP, replacing the stylet is a simple and effective means of lowering the incidence of MPH after LP.65 Given the innocuous nature of this maneuver, if no other prophylactic measures are taken, there appears to be little reason not to replace the stylet prior to epidural needle removal in the event of UDP.
Subarachnoid Saline
Limited evidence indicates that the subarachnoid injection of sterile preservative-free saline following UDP may be associated with a significant reduction in the incidence of MPH and the need for EBP. In one small study (n = 43), immediate injection of 10 mL saline through the epidural needle substantially reduced the incidence of MPH (32%, compared with 62% in a matched control group) and resulted in a significant reduction in the need for EBP (p = 0.004).73 The injection of saline and the reinjection of CSF have been speculated as important in the prevention of MPH by maintaining CSF volume.72 However, given the relatively rapid rate of CSF regeneration, it may be that the benefit of fluid injection following UDP is actually in preventing a wicking strand of arachnoid (as proposed for stylet replacement). Further investigation into this issue is needed.
Intrathecal Catheters
Immediately placing an intrathecal catheter (ITC) after UDP has the advantages of being able to rapidly provide spinal analgesia as well as eliminate the possibility of another UDP under challenging clinical circumstances. However, the potential benefits of ITC use must be weighed against the readily appreciated risks involved (accidental use, misuse, and infection). Although evidence is extremely limited, ITC use has also been proposed to reduce the risk of MPH after UDP.56 Ayad et al.74 placed and maintained an ITC for 24 hours following UDP. In their obstetric population, catheter placement resulted in an MPH rate of only 6.2%, with an expected incidence of >50% in this setting. A similar reduction in the development of MPH with 24-hour ITC maintenance after UDP has been noted in orthopedic patients.75 This impressive reduction in the incidence of MPH has generally not been reported from studies where catheters have been left in place for <24 hours. It has been proposed that the mechanism of benefit from ITC maintenance may be due to reaction to the catheter, with inflammation or edema preventing further CSF loss after removal. There are also preliminary data to suggest that the incidence of MPH may be further reduced by the injection of preservative-free saline through an ITC immediately prior to removal.73 With some accepted and other possible benefits, rates of ITC use following UDP have clearly increased during the past decade. Recent surveys of US, UK, and Australian practice have noted rates of routine intrathecal catheterization following UDP in obstetric patients of 18%, 28%, and 35%, respectively.15,64,76
Although ITC use has increased, reattempting an epidural at an adjacent interspace remains the preferred action following UDP.15 Provided an epidural catheter can be successfully placed, several epidural approaches have been used in the hope of reducing the incidence and severity of MPH:
Epidural Saline
Efforts have included both bolus (usually around 50 mL as a single or repeated injection) and continuous infusion techniques (commonly 600–1,000 mL over 24 hours). As these measures are resource intensive and may only serve to delay the inevitable onset of symptoms, they have generally not been continued beyond 36 hours. In one large analysis (n = 241), Stride and Cooper77 reported a reduction in the incidence of MPH from 86% in a conservatively treated control group to 70% with epidural saline infusion. Trivedi et al.78 noted a similar reduction in MPH (from 87% to 67%) in 30 patients who received a single prophylactic “saline patch” (40–60 mL) following completion of an obstetric procedure. Other studies of epidural saline have noted this modest decrease in the incidence of MPH. Stride and Cooper77 also reported a lower incidence of severe headache (from 64% to 47%), but this effect has been inconsistently seen by other investigators, and there is no convincing evidence that epidural saline reduces the eventual need for EBP.
Epidural Opiates
Epidural opiates (especially morphine), while long utilized for the treatment of MPH, have been thought unlikely to influence the natural history of the disorder. However, recently revisiting the issue of opiates as prophylaxis after UDP, Al-metwalli79 found that two epidural injections of morphine (3 mg in 10 mL), compared with epidural injections of an equal volume of saline, resulted in fewer episodes of MPH (p = 0.014) and decreased the need for EBP (p = 0.022). Due to the small number of patients involved (n = 25), further prospective investigation is warranted.
Prophylactic Epidural Blood Patch
The impressive efficacy of the EBP when used as treatment for MPH has fueled interest in the technique for prophylaxis. Research into the efficacy of the EBP for prophylaxis has yielded mixed results, and closer scrutiny indicates that optimism should be guarded. The strongest investigation to date has been by Scavone et al.,80 who performed a prospective, randomized, double-blind study in 64 parturients comparing the prophylactic epidural blood patch (PEBP) to a sham EBP. In this study, an identical 56% of patients in each group went on to develop MPH. Although there was a trend toward fewer therapeutic EBPs recommended and performed in the prophylactic group, the difference was not statistically significant (p = 0.08). The primary benefit of the PEBP was a shorter total duration of symptoms (from a median of ~5 days to 2 days) and, consequently, a reduction in the overall pain burden (Fig. 9-5).
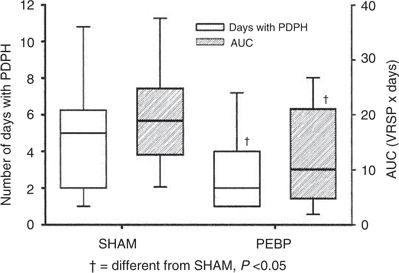
FIGURE 9-5. Plot of the duration of MPH (PDPH) (open boxes, with scale on left) and the pain intensity duration (shaded boxes, with scale on right) for sham injection and PEBP. Area under the curve (AUC) consists of the verbal rating score for pain (VRSP) multiplied by the number of days with MPH. Boxes are the interquartile range, solid lines within the boxes represent the median value, and whiskers are the 10th and 90th percentiles. (Used from Scavone BM, Wong CA, Sullivan JT, et al. Efficacy of a prophylactic epidural blood patch in preventing post dural puncture headache in parturients after inadvertent dural puncture. Anesthesiology 2004;101:1422–1427, with permission.)



