CHAPTER 14 Medical Management of Unstable Angina and Non–ST Segment Elevation Myocardial Infarction
Anti-ischemic Therapies
Anti-ischemic therapy is routine for all patients with ACS and has not changed significantly in the last several decades. It generally consists of nitrates (either sublingual or intravenous) and intravenous followed by oral administration of β blockade. In patients who cannot tolerate β blockade because of severe bronchospastic lung disease, the addition of a nondihydropyridine calcium antagonist can be considered (Table 14-1).
Table 14–1 Recommendations for Anti-Ischemic Therapy: Continuing Ischemia or Other Clinical High-Risk Features Present∗
| Bed/chair rest with continuous ECG monitoring |
| Supplemental oxygen with arterial saturation <90%, respiratory distress, or other high-risk features for hypoxemia. Pulse oximetry can be useful for continuous measurement of SaO2 |
| NTG 0.4 mg sublingually every 5 min for a total of 3 doses; afterward, assess need for intravenous NTG |
| Intravenous NTG for first 48 hr after UA/NSTEMI for treatment of persistent ischemia, HF, or hypertension |
| Decision to administer intravenous NTG and dose should not preclude therapy with other mortality-reducing interventions such as β blockers or ACE inhibitors |
| β blockers (via oral route) within 24 hr without a contraindication (e.g., HF) regardless of concomitant performance of PCI |
| When β blockers are contraindicated, a nondihydropyridine calcium channel blocker (e.g., verapamil or diltiazem) should be given as initial therapy in the absence of severe left ventricular dysfunction or other contraindications |
| ACE inhibitor (via oral route) within first 24 hr with pulmonary congestion, or LVEF ≤0.40, in the absence of hypotension (systolic blood pressure <100 mm Hg or <30 mm Hg below baseline) or known contraindications to that class of medications |
| ARB should be administered to UA/NSTEMI patients who are intolerant of ACE inhibitors and have either clinical or radiologic signs of HF or LVEF ≤0.40 |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; HF, heart failure; LVEF, left ventricular ejection fraction; NTG, nitroglycerin; PCI, percutaneous coronary intervention; UA/NSTEMI, unstable angina/non–ST segment elevation myocardial infarction.
∗ Recurrent angina or ischemia-related ECG changes (≥0.05 mV ST segment depression or bundle branch block), or both, at rest or with low-level activity; or ischemia associated with HF symptoms, S3 gallop, or new or worsening mitral regurgitation; or hemodynamic instability or depressed left ventricular function (LVEF <0.40 on noninvasive study); or serious ventricular arrhythmia.
Adapted from Anderson JL, Adams CD, Antman EM, et al: ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients with Unstable Angina/Non-ST-Elevation Myocardial Infarction) Developed in Collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol 2007;50:e1-e157.
Nitrates
Despite their routine use in patients with ACS, data showing that nitroglycerin reduces mortality or reinfarction are sparse.1,2 Nitroglycerin mainly provides symptom relief. Relief of symptoms is achieved by several mechanisms, including arterial vasodilation, reduction in coronary vasospasm, reduced myocardial oxygen demand (by decreasing preload and afterload), augmentation of collateral blood flow, and inhibition of platelet aggregation. There is no optimal intensity of nitroglycerin therapy, but a loading dose should be administered using sublingual nitroglycerin followed by either a topical or an intravenous agent. The dose is generally titrated until symptoms abate or side effects occur, most notably headache and hypotension. Caution should be exercised in patients who are volume depleted or relatively hypotensive or both because these patients may be sensitive to the preload reduction, and the beneficial effects of the nitrates may be outweighed by severe reduction in the mean arterial pressure.
β Blockers
In the absence of contraindications, all patients with ACS should be treated with intravenous β blockade as soon as possible to minimize the risk of arrhythmia or reinfarction.3–5 The cardioprotective effects of β blockers occur by reducing sympathetic activation and myocardial ischemia, attenuating the toxicity associated with high circulating catecholamines. β blockers decrease myocardial oxygen demand by reducing heart rate, contractility, and systolic blood pressure, and by prolonging diastolic filling, which may allow for increased coronary perfusion time. The antiarrhythmic effects of β blockers result from direct cardiac electrophysiologic activity with decreased spontaneous firing of ectopic pacemakers, slowed conduction, and increased refractory period of the atrioventricular node. Contraindications include β blocker allergy, bradycardia (heart rate <60 beats/min), heart failure, shock, and second-degree or third-degree atrioventricular block.
A meta-regression analysis of randomized controlled trials after myocardial infarction (MI) showed that β blockers reduce the odds of death in long-term trials by 23% and in short-term trials by 4%.6 In addition to the immediate administration of intravenous β blockade, patients who survive MI should be continued on oral agents indefinitely. A study in nearly 70,000 patients prescribed a β blocker (atenolol, metoprolol, or propranolol) after acute MI showed that these agents were associated with a 40% improvement in survival at 2 years, and suggested that the specific β blocker chosen has little influence on mortality.7
COMMIT has called into question the practice of routinely using intravenous β blockade in patients with ST segment elevation MI. Although benefits in reduction of reinfarction and ventricular fibrillation were noted, an excess of early shock was associated with use of intravenous β blockers.8 In UA/NSTEMI patients, the beneficial anti-ischemic and antiarrhythmic effects of intravenous β blockers may be counterbalanced by adverse effects in hemodynamic stability if administered rapidly and at high doses in patients with even mild heart failure or hypotension. In such patients, withholding β blockade 12 to 24 hours until hemodynamic stabilization has been confirmed may be prudent.
Calcium Antagonists
Apart from severe bronchospastic lung disease, allergy, and possibly cocaine ingestion, the contraindications for β blockers are the same as the contraindications for calcium antagonists. No definitive data exist to suggest that there are substantial reductions in ischemic complications of MI with these agents. A meta-analysis of trials of patients with acute MI or UA who were treated with calcium antagonists found that these agents do not reduce the risk of initial or recurrent infarction or death,9 reinforcing the fact that β blockers are the preferred initial therapy, and calcium channel blockers should not be routinely used as first-line therapy for ACS.
Antiplatelet Therapy
Circulating platelets play a crucial role in normal hemostatic function; however, platelet aggregation and thrombosis are maladaptive processes in ACS. Atherosclerotic plaque rupture during ACS exposes thrombogenic subendothelial components, leading to platelet deposition and activation.10 Platelet activation is associated with surface expression of greater numbers of glycoprotein (GP) IIb/IIIa receptors that are available for binding fibrin strands and platelet cross-linking. In addition to cross-linking as part of the aggregation process, activated platelets also release local mediators that can induce further platelet accumulation and activation, vasoconstriction, thrombosis, and mitogenesis. Platelet activation leads not only to thrombus formation in the epicardial artery, but also distal embolization and occlusion of the microcirculation, which have been associated with poor outcomes.11,12 Because of the significant contribution of platelets to the pathophysiology of ACS, one of the cornerstones of medical therapy is directed at inhibiting their deleterious effects.
Aspirin
Numerous trials consistently show the benefits of aspirin in patients with ACS,13–18 and its use has become the foundation of medical management for UA/NSTEMI. Aspirin acts by inhibiting cyclooxygenase-1 within platelets, which interferes with the biosynthesis of thromboxane A2, prostacyclin, and other prostaglandins. These prostanoids are the main determinants of platelet aggregation in this pathway.
Acute treatment with aspirin is recommended for all patient with suspected ACS, unless contraindicated, and should be continued indefinitely. Current American College of Cardiology/American Heart Association (ACC/AHA) guidelines recommend an initial dose of aspirin of 162 to 325 mg.2 In clinical practice, most physicians in the United States use initial doses of aspirin of 325 mg during the initial phase of ACS. After stent implementation, a maintenance dose of 325 mg is recommended for 1 month with bare metal stents and for 6 to 12 months with drug-eluting stents. Thereafter, or in the absence of percutaneous coronary intervention (PCI), the optimal maintenance dose of aspirin is 75 to 162 mg daily.2 Ibuprofen interferes with the cardioprotective effects of aspirin19; an alternative nonsteroidal anti-inflammatory drug ideally should be prescribed to patients requiring indefinite aspirin therapy. If ibuprofen use cannot be avoided, it should be taken at least 30 minutes after or at least 8 hours before aspirin.2
There is growing evidence that many individuals may exhibit aspirin resistance.20 Whether the term aspirin resistance represents a clinical failure of aspirin therapy (i.e., stroke, MI) or some level of residual platelet aggregation despite therapy remains a matter of debate. Nevertheless, this syndrome has been variously described as relative failure to inhibit platelet aggregation or failure to prolong bleeding time, or the development of a clinical event while on aspirin therapy. Presently, no test of platelet function is currently preferred to assess the antiplatelet effect of aspirin in the individual patient because it is difficult to assess the clinical significance of in vitro platelet function studies. In patients with contraindications or intolerance to aspirin or patients who experience a cardiac event while taking aspirin (e.g., aspirin failures), clopidogrel, 75 mg daily, is a suitable alternative.
Thienopyridine Agents
The widespread use of clopidogrel in the treatment of ACS is based primarily on the CURE and CREDO trials.21,22 The CURE trial compared the benefit of aspirin plus clopidogrel (300 mg loading dose, then 75 mg/day) treatment versus aspirin alone in 12,562 patients with UA/NSTEMI for 3 to 12 months. The use of clopidogrel reduced the risk of cardiovascular death, recurrent MI, or stroke significantly by 20%, from 11.5% in the placebo group to 9.3% in the clopidogrel group. A reduction of death, nonfatal MI, stroke, or refractory or severe ischemia was already present within 24 hours of randomization.21 The beneficial effects of clopidogrel were noted across all subgroups regardless of whether high-risk features were present, such as electrocardiogram (ECG) changes or elevated cardiac biomarkers, suggesting broad use for this agent as adjunctive therapy in all UA/NSTEMI patients if no contraindications exist.
In PCI-CURE, an observational substudy of the CURE trial, 2658 patients underwent PCI a median of 10 days after randomization.23 All patients received an open-label thienopyridine after PCI for approximately 30 days, after which the original blinded study drug was restarted for an additional 8 months. Pretreatment with clopidogrel was associated with a 30% reduction in risk of cardiovascular death, recurrent MI, or target vessel revascularization at 30 days (6.4% versus 4.5%). The benefit of pretreatment and long-term therapy with clopidogrel before PCI was also observed in the CREDO trial.22 CREDO comprised 2116 patients, 55% of whom had UA/NSTEMI and were to undergo PCI, and compared pretreatment with clopidogrel, 300 mg, followed by maintenance therapy, 75 mg, versus placebo with 1-year follow-up. Patients allocated to placebo received 75 mg of clopidogrel at the time of PCI, followed by 75 mg daily for 1 month and placebo thereafter. The benefit of pretreatment with 300 mg of clopidogrel was primarily observed among patients who had received pretreatment 15 hours or more before PCI.24
Taken together, the PCI-CURE and CREDO trials suggest that ACS patients should be treated early with clopidogrel before PCI, and treatment should be continued for 9 to 12 months. A suggested algorithm for antiplatelet therapy after an episode of UA is outlined in Figure 14-1.
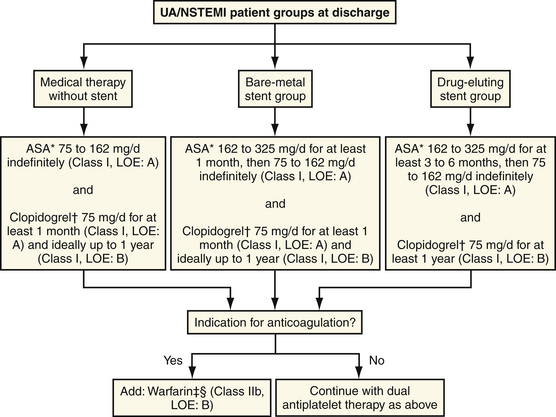
(Adapted from Anderson JL, Adams CD, Antman EM, et al: ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients with Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol 2007;50:e1-e157.)
More recent investigation in this area has focused on the optimal timing and loading dose of clopidogrel before PCI. ARMYDA-2 randomly assigned patients to receive either a 300-mg or 600-mg loading dose of clopidogrel 4 to 8 hours before PCI, and found that the 600-mg loading dose significantly reduced periprocedural MI.25 Most patients in ARMYDA-2 underwent PCI for stable angina (75%). Cuisset and colleagues26 randomly assigned 292 consecutive patients with non–ST segment elevation ACS undergoing stenting to receive a 300-mg or 600-mg loading dose of clopidogrel at least 12 hours before PCI. During the 1-month follow-up, 18 (12%) cardiovascular events occurred in the 300-mg group compared with 7 (5%) events in the 600-mg group. Based on the current available evidence, all patients without contraindications who present with ACS should be given a 300-mg loading dose of clopidogrel, and in situations in which longer durations of pretreatment are impossible, 600 mg may be used when given at least 2 hours before PCI.2
Several new thienopyridine agents are currently being investigated. Prasugrel is a thienopyridine that has been shown in preclinical studies to be more potent and to have a more rapid onset of action than clopidogrel.27 It was evaluated in the TRITON-TIMI 38 study, in which 13,608 ACS patients undergoing PCI were randomly assigned to prasugrel or clopidogrel. Although prasugrel therapy was associated with significantly reduced rates of ischemic events, this benefit came at a cost of more major bleeding events.28 AZD-6140, another newer thienopyridine with greater potency than clopidogrel, is currently being evaluated. Cangrelor, an intravenous agent with an extremely short half-life and rapid onset of action,29 is the subject of the ongoing CHAMPION-PLATFORM and CHAMPION-PCI trials.
Glycoprotein IIb/IIIa Receptor Inhibitors
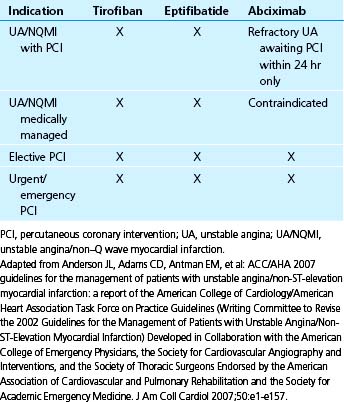
Platelet activation is associated with surface expression of greater numbers of GP IIb/IIIa receptors available for binding fibrin strands and platelet cross-linking. Several large, randomized clinical trials have shown improved clinical outcomes with the administration of GP IIb/IIIa receptor inhibitors (GPIs) in patients presenting with ACS and after PCI to inhibit platelet aggregation.30 Although intravenous agents seem to be beneficial, the oral GPI, in contrast, has been ineffective and may increase mortality.31 Three intravenous GPIs are currently available for clinical use: abciximab, tirofiban, and eptifibatide. Abciximab is a GPI that comprises Fab fragment of the chimeric human-murine monoclonal antibody 7E3. Tirofiban is a nonpeptide inhibitor of the platelet GP IIb/IIIa receptor, interfering with aggregation by mimicking the geometric, stereotactic, and charge characteristics of the platelet integrin-binding domain sequence. Eptifibatide is a nonimmunogenic, cyclic heptapeptide with an active pharmacophore that is derived from the structure of barbourin, a platelet GPI from the venom of the Southeastern pigmy rattlesnake.32 Table 14-2 summarizes the clinical indications for GPI administration.
The TIMI risk score for UA/NSTEMI33 (Fig. 14-2) and GRACE prediction score34 (Fig. 14-3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree