CHAPTER 29 Acute Aortic Syndromes
Diagnosis and Management
Acute Aortic Dissection
Acute dissection of the thoracic aorta is one of the most common catastrophic aortic conditions encountered in clinical practice. The incidence of aortic dissection has been reported to be approximately 2.9/100,000/yr.1 The variable clinical presentations of aortic dissection, in combination with a mortality rate in untreated cases as high as 1% per hour during the first 48 hours after the onset of symptoms, underscore the importance of a high index of suspicion and prompt diagnosis and therapy.2–4 Noninvasive testing (TEE, CT, and MRI) allows an accurate diagnosis to be made in the majority of patients.5 Effective treatment exists so that future improvements in initial and long-term survival in acute aortic dissection depend on increased clinical awareness, rapid noninvasive diagnosis, and the early institution of appropriate medical and/or surgical therapy.
Pathogenesis
Aortic dissection originates at the site of an intimal tear in more than 95% of patients.2,6 The resultant intimal tear exposes the media to pulsatile aortic flow, creating a second or “false” aortic lumen that then dissects in the outer layer of aortic media, propagating distally and, occasionally, proximally. One feature differentiating proximal and distal aortic dissection is the greater frequency of hypertension in patients with distal dissection, a possible factor in pathogenesis.7
The primary intimal tear is located, in decreasing order of frequency, in the ascending aorta 1 to 5 cm above the right or left sinus of Valsalva (65%), in the proximal descending thoracic aorta just beyond the left subclavian artery origin (20%), in the transverse aortic arch (10%), and more distally in the thoracic aorta or abdominal aorta (5%).2,6
Once initiated, the dissection usually extends distally and, occasionally, proximally for a variable distance. As the dissecting process encounters branches of the aorta it may pass around their origins, extend into their walls, or occlude them.2,6 Reentry of the dissection through a second, more distal intimal tear may occur, usually in one of the iliac arteries. External rupture of the dissecting process into the pericardial space is the most common cause of death in aortic dissection; congestive heart failure, usually due to aortic regurgitation, is the second most common cause of death.
Predisposing Factors
Aortic dissection most frequently affects patients in the fifth to seventh decades of life (mean age 63 years) and is more common in men (65.3% male).8 In patients younger than 40 years, the incidence between men and women is equal due to the occurrence of aortic dissection in women during pregnancy, with approximately 50% of all aortic dissections occurring during the third trimester of pregnancy.9
Systemic hypertension is the most frequently encountered predisposing factor for aortic dissection, being present in more than 70% of patients.2,3,10–12 Hypertension may play a role in initiating the intramural hematoma along with having a direct weakening effect on the aortic media.6,12 The causative role of systemic hypertension is further supported by the finding that coarctation of the aorta predisposes to aortic dissection.13
Other major risk factors for aortic dissection, especially proximal aortic dissection, are congenitally bicuspid or unicommissural aortic valves,10,12 and the congenital disorders of connective tissue such as Marfan syndrome14 and Ehlers-Danlos syndrome. Additional predisposing factors for acute aortic dissection include pre existing aortic aneurysm15 and a positive family history (as many as 19% of patients).
Iatrogenic aortic dissection is an uncommon but potentially serious complication of invasive angiographic procedures and cardiac surgery. Angiographic procedures such as coronary angiography, percutaneous transluminal coronary angioplasty, intra-aortic balloon insertion, and translumbar aortography have been complicated by aortic dissection.12,16–19 Catheter-induced dissection can originate at any location, and it is most common in the sinus of Valsalva, brachiocephalic arteries, descending thoracic aorta, abdominal aorta, and iliac or femoral arteries. The majority of catheter-induced dissections are retrograde dissections and tend to decrease in size over time due to thrombosis of the false lumen, whereas anterograde dissections tend to persist on follow-up. Nearly all iatrogenic aortic dissections can be treated medically with serial clinical examinations and noninvasive testing used to identify those in need of surgical therapy.17
Cardiac surgical procedures complicated by aortic dissection include those that require cross-clamp or cannulation of the ascending aorta, such as aortic valve replacement12,20,21,15 and/or coronary artery bypass grafting.12,22,23 Dissection can arise at the site of ascending aortic cannulation, aortosaphenous vein anastomosis, aortic cross-clamp, or as a result of direct arterial injury. Dissection in association with these procedures usually occurs intraoperatively and is promptly diagnosed and treated, but chronic dissection in the postoperative period has been reported.21
Aortic dissection has also been reported in association with inflammatory diseases (giant cell aortitis,12,24 Takayasu aortitis, rheumatoid arthritis, syphilitic aortitis, systemic lupus erythematosus,25 Noonan syndrome,26 Turner syndrome,27 fibromuscular dysplasia,11 annuloaortic ectasia,11 aortic coarctation, cocaine use,28,29 methamphetamine use, polycystic kidney disease,30 polyarteritis nodosa,31 trauma,32 high-intensity weight lifting.33
Classification
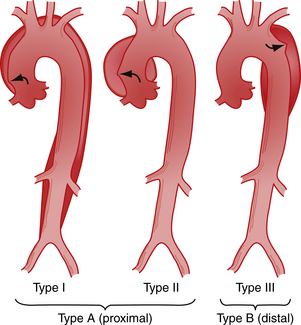
There are two different anatomic systems—the DeBakey and Daily (Stanford) systems—used to classify aortic dissection. The DeBakey system34 is based on the site of origin of the dissection and recognizes three types of dissection (Fig. 29-1): type I—the dissection arises in the ascending aorta and extends distally; type II—the dissection is limited to the ascending aorta; and type III—the dissection arises at or just distal to the origin of the left subclavian artery and extends distally or, rarely, retrogradely into the arch of the ascending aorta.
For clinical purposes, because types I and II have a similar prognosis, aortic dissection can be broadly divided into those with the tear in the ascending aorta and those with the tear in the descending aorta, also called “proximal” and “distal,” respectively.35 A similar classification system, proposed by Daily and associates,36 designates all dissections involving the ascending aorta as type A, regardless of the site of the primary intimal tear. All other dissections are designated type B.
An important aspect of classification is the duration of the dissection at the time of first presentation. Dissections are categorized as “acute” if the diagnosis is made within 2 weeks of symptom onset and as “chronic” if more than 2 weeks have elapsed. The distinction is important due to the fact that approximately 65% to 75% of patients with untreated aortic dissection die in the first 2 weeks after the onset of symptoms.2
Intimal tear without hematoma is an uncommon variant of aortic dissection characterized by a localized intimal tear exposing the underlying media or adventitial layers to pulsatile aortic flow. There is no progression or separation of the medial layers.37
Clinical Features
Due to variable involvement of the aorta and its branches by the dissecting process, the patient with acute aortic dissection may have clinical manifestations of ischemia of various organ systems, singly or in combination, and symptoms and signs of cardiac disease. The diverse presentations of aortic dissection can provide for difficult diagnosis, and misdiagnosis commonly occurs.2,12 Despite major advances in the noninvasive diagnosis of aortic dissection and in medical and surgical therapy, up to 55% of patients in reported series die without a correct antemortem diagnosis.1,2,12,38,39 For these reasons, a high index of clinical suspicion for acute aortic dissection in any likely setting is imperative.
The classic presentation of a patient with acute aortic dissection, occurring in more than 70% of patients, is the sudden onset of severe pain, usually beginning in the anterior chest, radiating to the back, and moving distally as the dissection progresses.1,35 The pain is most commonly described as sudden in onset with a “ripping,” “tearing,” or “stabbing” quality; this is in contrast to the crescendo nature of the discomfort of acute myocardial infarction. Chest pain is significantly more common in patients with proximal (type A, type I, and type II) dissection (79% versus 63% in distal [type B, type III] dissection). In contrast, both back pain (64% versus 47%) and abdominal pain (43% versus 22%) are significantly more common with distal (type B) aortic dissection.8 Patients with acute aortic dissection often appear to be in shock, although blood pressure is elevated in one half to two thirds of cases, especially with distal dissection.8 Hypotension in aortic dissection is usually attributable to rupture of the dissection into the pericardial space with resultant cardiac tamponade or, less commonly, the pleural space or mediastinum and is more common with proximal dissection.
It should be remembered, however, that painless dissection occurs in 14% to 21% of patients.2,12,40 Patients with painless aortic dissection tend to be slightly older, have a prior history of diabetes, aortic aneurysm, or prior cardiac surgery, and more often have type A dissection. Syncope, congestive heart failure, or stroke are also more common in this group. Importantly, in-hospital mortality was significantly higher than for patients having pain (33% versus 23%).8
A cardiac murmur may be present, usually at the cardiac base, and may be systolic, diastolic, or both. A diastolic decrescendo murmur of aortic regurgitation indicates involvement of the ascending aorta and is heard in one half to two thirds of patients with type A aortic dissection.8 Congestive heart failure, when present in association with proximal aortic dissection, is most often due to severe aortic regurgitation,10,35 but cases of congestive heart failure due to rupture of the dissecting process into the right or left atrium or right ventricle have also been reported.41–43 Myocardial infarction, most commonly inferior infarction, occurs in 1% to 2% of patients and is due to compromise of the coronary ostium by a hematoma or intimal flap. Peripheral pulse deficits are noted in 19% to 30% of patients,3,8,35 more commonly with proximal aortic dissection, and is associated with a higher rate of in-hospital complications and mortality.44 Pulse deficits may be transitory due to oscillation of the intimal flap or distal reentry of the hematoma into the true lumen. Acute lower extremity ischemia, with or without chest pain, as a result of dissection extending into the iliac arteries occurs in 6% to 12% of patients,3,45,46 and may provide an important clue to the diagnosis.45 Other cardiovascular findings include a difference in systolic blood pressure between the arms (>20 mm Hg), tachycardia, friction rubs, bruits, pulsus paradoxus, and cardiac tamponade.
Syncope in a patient with aortic dissection is an important event as it is associated with a worse prognosis. Syncope most commonly results from rupture of the dissecting process into the pericardial space producing cardiac tamponade.12,35 Less commonly, rupture occurs into the left pleural space producing a left hemothorax.12 Neurologic deficits, including cerebrovascular accident, disturbances of consciousness, ischemic paraparesis, and ischemic peripheral neuropathy, occur in more than 40% of patients, more commonly with proximal aortic dissection.1,47
Other less frequent findings that occur in association with acute aortic dissection include Horner syndrome, a pulsatile sternoclavicular joint,48 vocal cord paralysis, hemoptysis,49 superior vena cava syndrome,50 upper airway obstruction,51 hematemesis,52 pleural effusion, unilateral pulmonary edema,53 signs of mesenteric or renal infarction, fever,54 and deep venous thrombosis.12
Diagnosis
Aortic dissection is usually suspect from the initial history and physical examination. The majority of patients with aortic dissection can be identified based upon some combination of three clinical factors: (1) sudden onset of chest or abdominal pain with a sharp, tearing, and/or ripping character; (2) mediastinal and/or aortic widening on chest radiograph; (3) a pulse variation (absence of a proximal extremity or carotid pulse) and/or blood pressure (>20 mm Hg difference between the arms).55 The incidence of aortic dissection was 83% when a pulse or blood pressure differential or any combination of the three variables occurred.
Routine laboratory studies are nonspecific and play a minor role in the initial assessment of patients with suspected acute aortic dissection. Their main value lies in the exclusion of other diseases. A mild to moderate leukocytosis occurs in two thirds of patients, and anemia may occur as a result of leakage of the dissection or from sequestration of blood in the false lumen. Serum lactate dehydrogenase and bilirubin levels are sometimes increased because of blood sequestered within the false lumen, but serum glutamic oxaloacetic transaminase and creatine phosphokinase are usually normal.35 Hematologic studies consistent with disseminated intravascular coagulation can also occur. In one small study, D-dimer was positive in all patients with proximal aortic dissection, suggesting a negative test makes the presence of disease unlikely.56
The most common electrocardiographic abnormality in patients with aortic dissection is left ventricular hypertrophy from chronic systemic hypertension.3 Acute electrocardiographic changes occur in up to 55% of patients and include ST segment depression, T-wave changes, and ST segment elevation, in decreasing order of frequency.57 Acute ischemic changes can occur when one or both coronary ostia become obstructed, either by the intimal flap or from external compression by the dissecting hematoma. The electrocardiographic changes of acute pericarditis may be seen if there has been leakage of blood into the pericardial space. Heart block resulting from proximal extension of the hematoma into the area of the atrioventricular node has also been reported.58 The chief value of the electrocardiogram is in distinguishing aortic dissection from acute myocardial infarction, although the two conditions can coexist.
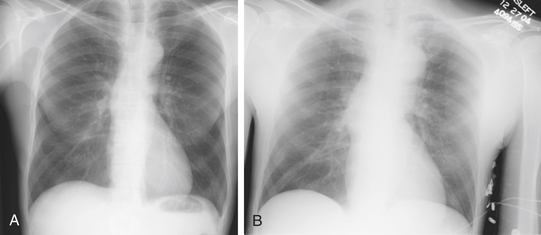
Chest radiography may be helpful in suggesting the diagnosis of aortic, with abnormalities of the aortic dissection silhouette being the most common finding.8,59 Additional findings include pleural effusion, mediastinal widening, displacement of intimal calcification greater than 6 mm inside the outer edge of the aortic shadow, and the radiographic findings of congestive heart failure (Fig. 29-2). Nonetheless, it is important to remember that normal chest radiographic findings (present in 11% to 16% of patients) do not exclude the diagnosis of aortic dissection.8
Currently available noninvasive imaging modalities that are accurate in the diagnosis of acute aortic dissection include echocardiography (combined transthoracic echocardiography [TTE] and TEE), CT, and MRI. The choice of test depends on which imaging modality is most readily available at a particular institution and the hemodynamic stability of the patient. Most patients have multiple imaging studies performed; in the initial IRAD review, the initial study performed was CT in 61%, echocardiography in 33%, aortography in 4%, and MRI in only 2%.8
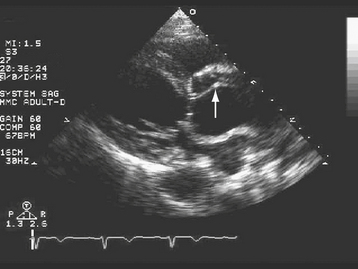
TTE can be very useful in some patients with suspected aortic dissection (Fig. 29-3). When the findings of a dilated aortic root (end-diastolic diameter >42 mm), widening of the aortic walls (16 to 20 mm for the anterior wall and 10 to 13 mm for the posterior wall), and a linear undulating echo representing the intimal flap are present, the positive predictive value for TTE is 100%.60–62 Advantages of TTE are its portability, rapid “online” diagnosis, and ability to identify associated aortic regurgitation and other valvular disease, and to assess left ventricular function, regional wall motion abnormalities, pericardial effusion, and cardiac tamponade. The diagnosis of cardiac tamponade in a patient suspected of having aortic dissection deserves special mention. Echocardiographically guided pericardiocentesis should be avoided in this setting because the rapid withdrawal of pericardial fluid can result in a prompt improvement in left ventricular systolic function, left ventricular dP/dT, and systolic blood pressure, producing aortic rupture.63,64 The patient with aortic dissection complicated by cardiac tamponade should be taken emergently to surgery for institution of cardiopulmonary bypass followed by evacuation of blood from the pericardial space. Disadvantages of TTE limiting its utility in the diagnosis of aortic dissection include difficulty in adequately visualizing the descending thoracic aorta,5 and in patients with suboptimal echocardiographic “windows” due to obesity or chronic obstructive pulmonary disease. Overall, the sensitivity and specificity of TTE are inferior to TEE, CT, and MRI in the diagnosis of aortic dissection.6
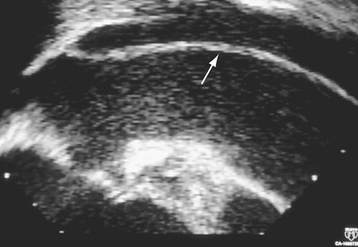
Multiplane TEE, in combination with TTE, has a sensitivity of 99% and a specificity of 98% in the diagnosis of aortic dissection.65 TEE is portable, minimally invasive, and can accurately determine the type and extent of dissection safely in an emergent setting, allowing rapid triage of patients to either surgical or medical therapy66,67 (Fig. 29-4). Color flow Doppler imaging significantly improves the sensitivity of TEE by allowing visualization of the intimal flap, dissection entry site, the true and false lumens, the presence of thrombus, associated aortic regurgitation, and the proximal coronary arteries67 (see Fig. 29-2). In addition, multiplane TEE provides a comprehensive assessment of left ventricular systolic function, regional wall motion, pericardial effusion, and cardiac tamponade. The overall sensitivity of TEE is comparable with CT,68 MRI,5,69,70,71 and aortography72 in the diagnosis of aortic dissection. Current multiplane TEE probes have largely overcome impediments in the ascending aorta having monoplane and biplane transducers, although artifacts in this region continue to be a diagnostic challenge.
CT with intravenous iodinated contrast enhancement is also an accurate noninvasive screening test in patients with suspected aortic dissection.73 Advantages of CT include ready availability at most hospitals, even on an emergency basis, and improved accuracy with spiral (helical) CT and with electron beam (ultrafast) or multidetector (multislice) CT.74
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree