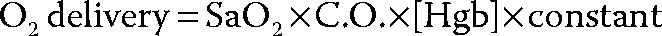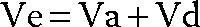CHAPTER 50 Mechanical Ventilation in the Cardiac Care Unit
Introduction
The history of mechanical ventilation is replete with famous figures in pulmonary physiology, anesthesia, and surgery.1 Ventilators have been used in the operating room since the end of the 1900s, but widespread use in respiratory failure began in the 1950s treating patients with paralysis from polio. Advances in oxygen delivery and blood gas analysis allowed the treatment of patients with increasingly complex pulmonary and chest wall diseases over the next decade. Modern ventilators continue to advance with improvements in reliability and responsiveness and increasing ability to control all phases of the inspiratory cycle.
Impact of Positive Pleural Pressure on Hemodynamics
Transitioning from negative pressure in the pleura to positive has important hemodynamic consequences affecting both the right- and left-sided cardiac circulations.2 A complete review of this topic is beyond the scope of this chapter, but understanding basic cardiopulmonary interactions allows the reader to predict the impact of positive pressure breathing on a given patient’s condition.3,4
Positive alveolar pressure affects blood circulation in two important ways:
The interplay of breathing and hemodynamics offers an opportunity to determine preload responsiveness in critically ill patients. Combining the models of Guyton and Starling, Magder and colleagues showed that careful interpretation of the central venous pressure waveform during spontaneous breathing can differentiate those patients who are on the ascending portion of their Starling filling curves, thereby correctly predicting which patients will increase their cardiac output with a fluid challenge.8,9 Similarly patients with large swings in arterial blood pressure with positive intrathoracic pressure are preload dependent and likely responsive to fluid.10 A thorough review of this topic has been published.11
Indications for Mechanical Ventilation
Other indications for mechanical ventilation in the CCU include:
Managing Gas Exchange and Oxygen Delivery
Oxygenation
Importantly, the 3 factors in the equation above can interact with each other; for example increasing the intensity of mechanical ventilation to improve SaO2 may over-pressurize the thorax, reduce venous inflow to the right ventricle and impair cardiac output. Excessive red blood cell transfusion to achieve a “normal” hemoglobin concentration is also ill advised, as it will increase blood viscosity and may impair C.O. Careful attention to these interactions will guide the clinician in choosing interventions to improve O2 delivery to tissues.
Severe hypoxemia however may be refractory to increases in FiO2 when it is due to pulmonary “shunting,” in which a significant fraction of the cardiac output passes through a lung capillary bed that is not aerated (not exposed to gas). This commonly occurs in patients with consolidated pneumonia, massive pulmonary edema or atelectasis due to airway obstruction or compression of lung tissue. In these scenarios, even FiO2 levels approaching 100% will be unsuccessful in treating the cyanosis, just as in patients with an intra-cardiac shunt. Fortunately, there are alveoli at the margins of consolidated or atelectatic lung regions that can be “recruited” to function as gas exchange units if a high distending pressure is maintained. These higher airway pressures are commonly achieved by applying positive end-expiratory pressure (PEEP), which exerts its beneficial effect on PaO2 by preventing the collapse of recruitable lung units during exhalation. The effect of PEEP on cardiac output is complex, as it reduces both RV preload and LV afterload. The result may be either beneficial or deleterious, depending on the patient’s volume status and lung compliance.12,13 This is detailed in the preceding section.
Ventilation
The chief reason to control alveolar ventilation or PaCO2 is to regulate pH. Marked acidosis can impair cardiac contractility and responsiveness to vasopressors14,15; marked alkalosis reduces the threshold for ventricular arrhythmias in susceptible patients.16 However mild derangements in pH (0.10 to 0.15 units either way) are generally harmless. Thus strict control of pH by adjusting Ve is often unnecessary, particularly if it requires harmful increases in the intensity of alveolar ventilation (see section on ARDS, below). Inadequate levels of Ve will however cause respiratory acidosis (low pH, high PaCO2), stimulating the patient’s respiratory drive; in experimental studies this may divert up to 30% of cardiac output to the respiratory muscles.17 Conversely, excessive Ve causes respiratory alkalosis (high pH, low PaCO2) which suppresses respiratory drive and can cause a patient to be apneic during a weaning trial, thereby prolonging the use of mechanical ventilation.
Delivery of Mechanical Ventilation
There is a rapidly expanding menu of options when delivering mechanical ventilation. Terminology is complicated by proprietary names entering bedside usage and by the retention of historical abbreviations that were nonideal. Although nomenclature standardization has been proposed, it has not reached universal usage, causing confusion at the bedside.18,19 Another source of confusion is that different ventilators may require entry of a setting in different ways; for example, inspiratory time may be entered in seconds (e.g., 1.5 seconds) or as a percentage of cycle time (e.g., 25% of 6 seconds) or a ratio of inspiration to expiration (e.g., 1:3 of 6 seconds).
Despite the rapid increase in options, there are little randomized controlled data showing a benefit to a particular style of ventilation. There are significant differences in comfort and muscle unloading depending on the mode selected, but no mode has been shown superior in regards to patient outcome.20,21 Ventilator setting in most ICUs remains dependent on local expertise and the model of mechanical ventilator available. Improved understanding of the available modes allows finer control over ventilation and a better understanding of how a change in patient status will affect the setting. Most important to the patient is whether ventilation is assured, the respiratory muscles are effectively unloaded, and whether the tidal volume and end-expiratory volume are appropriate for the disease.
Mechanical ventilators allow manipulation of nearly every aspect of inspiration; expiration however, is not controlled except for airway pressure (PEEP). Usually discussed first is the pattern of breathing, or mode. A mode will allow different types of breath: each with its own name, options, and settings. These issues will be introduced in this chapter but full discussion requires a textbook and is beyond the scope of this chapter.22
Most breaths on mechanical ventilation can be classified as either mandatory or spontaneous. The ventilator exerts more control during mandatory breaths; settings include when to start the breath, what to control during the breath, and when the breath ends. Common mandatory breaths include: volume control, pressure control, and demand-flow volume control. A hallmark of mandatory breaths is the required setting of inspiratory time. During spontaneous breathing, the ventilator does not control inspiratory time but rather supports the inspiratory efforts. Common spontaneous breaths include pressure support and unsupported.
Some ventilators allow breaths that are not easily classified as mandatory or spontaneous. One example is volume-assured pressure support, which is a hybrid with spontaneous breathing that converts to mandatory if a tidal volume target is not reached.23 Another example is biphasic positive airway pressure (BIPAP) in which there are two asynchronous cycles as the ventilator maintains a background pattern of varying positive pressure breathing interspersed with patient effort.24
Mode
Generally the first decision in ventilator setting is the mode. The most common modes are continuous mandatory ventilation (CMV), intermittent mandatory ventilation (IMV), and spontaneous ventilation (CPAP).18 CPAP, standing for continuous positive airway pressure, describes a mode which is continuously spontaneous and therefore never mandatory. The name is intrinsically confusing, as the acronym CPAP also signifies a therapy for sleep apnea, a type of mask, and delivery of positive airway pressure. It is also inaccurate as airway pressure is not constant (when used in conjunction with pressure support) or necessarily positive as CPAP can be set to zero. Despite attempts at renaming, CPAP remains entrenched in bedside and ventilator manufacturer usage.
IMV delivers a set number of mandatory breaths delivered per minute. Patient triggering above this set rate delivers spontaneous breaths. As such, IMV requires setting of two distinct parameters: a full description of the mandatory breaths and the level of support to deliver during spontaneous breaths. Careful attention to both the mandatory and spontaneous breaths is needed to appropriately adjust the amount of ventilatory support delivered to the patient. Most ventilators attempt to synchronize the mandatory breaths with patient effort so a patient trigger may yield a mandatory or a spontaneous breath. This can lead to instability in the respiratory center as identical phrenic nerve output results in different tidal volumes. During IMV, patients may be performing more work during the mandatory breaths than during the spontaneous ones.25
Mandatory Breaths
Volume Control
Flow is predetermined and set during these breaths. Some ventilators require inspiratory flow to be constant (“square”); others allow different shapes such as decelerating (“ramp”). After normalization for inspiratory time, the impact of changing shape is likely small.26,27 Although certain ventilators appear to have different setting requirements, delivery of a volume control breath includes these presets: flow, volume, and time. Inspiratory time is determined by the time required to deliver the set volume at the set flow rate and shape.
It should be clear that volume control requires careful attention to flow rate settings. Settings need to satisfy the patient’s need for airflow and maintain an appropriate inspiratory time to allow expiration. Practitioners at the bedside need to readjust flow settings to changing patient effort such as occurs during awakening from sedation.
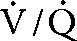 ) mismatch causing severe hypoxemia, and increased work of breathing causing ventilatory failure and CO2 retention. Excess work of breathing increases the oxygen demand by the diaphragm, further stressing the heart. In this state, the goals of mechanical ventilation are to improve gas exchange and reduce the patient’s work of breathing. Settings for mechanical ventilation should reflect these dual goals.
) mismatch causing severe hypoxemia, and increased work of breathing causing ventilatory failure and CO2 retention. Excess work of breathing increases the oxygen demand by the diaphragm, further stressing the heart. In this state, the goals of mechanical ventilation are to improve gas exchange and reduce the patient’s work of breathing. Settings for mechanical ventilation should reflect these dual goals.