Body weight (kg)
Targeted increase in MCF (A10) FIB TEM
4 mm
8 mm
12 mm
16 mm
20
0.5 g
1 g
1.5 g
2 g
40
1 g
2 g
3 g
4 g
60
1.5 g
3 g
4.5 g
6 g
80
2 g
4 g
6 g
8 g
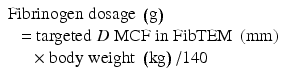
TEG monitoring can reduce transfusion requirements by 30 % [119]. TEG/ROTEM might improve patient outcomes: however, larger trials are required to investigate/demonstrate this hypothesis [144].
TEG/ROTEM can identify hyperfibrinolysis, indicating antifibrinolytic therapy in case of bleeding (Table 10.2).
Table 10.2
Simplified summary of three published TEG-based transfusion algorithm on kaolin blood
11 min ≤ r-time ≤ 15 min | 40 ≤ MA ≤ 46 mm and FF-MA 7–19 mm | 40 ≤ MA ≤ 46 mm and FF-MA 7–19 mm | alpha angle <52° | Ly 30 min > 8 % |
10 mL/kg FFP or 2 U FFP | Apheresis platelets 1U | Fibrinogen 25–50 mg/kg or Cryoprecipitate 6U | 2 UI FFP or fibrinogen concentrate | Tranexamic acid 1–2 g |
r-time > 15 min | MA < 40 mm and FF-MA 7–19 mm | MA < 40 mm and FF-MA ≤7 mm | alpha angle <45° | Ly 30 min >8 % |
20 mL/kg FFP or 4 U FFP | Apheresis platelets 2 U | Fibrinogen 25–50 mg/kg or Cryoprecipitate 6 U | Fibrinogen 25–50 mg/kg or Cryoprecipitate, 6 U | Tranexamic acid 1–2 g |
Every therapeutic decision (FFP, PLT, fibrinogen administration) should be done in case of ongoing bleeding and should be verified by a new TEG test (Table 10.3).
Table 10.3
Simplified summary of ROTEM-based transfusion algorithm
EXTEMCT >80 s | INTEM CT >240 s | EXTEM MCF <35 mm and FIBTEMMCF >6 mm | EXTEM MCF <35 mm and FIBTEMMCF <6 mm | EXTEM and INTEM decline after MCF APTEM normal CLI30EX <50 % |
PCC 25 UI/kg | 15–20 mL/kg FFPa | Apheresis platelets,1 U | Fibrinogen 25–50 mg/kg | Tranexamic acid 1–2 g or 25 mg/kg |
10.10 TEG-/ROTEM-Based Pharmacological Management of Bleeding
The management of bleeding and coagulopathy varies greatly between different centers. Again, VET devices offer a rapid diagnostic point-of-care test to aid the clinician in orienting/directing pharmacological therapeutic interventions.
10.10.1 Antifibrinolytic Drugs
Hyperfibrinolysis may play a significant role in nonsurgical blood loss requiring massive transfusion during liver transplantation. Antifibrinolytic therapy has been shown to reduce blood loss and blood product transfusions [102, 146] and to be a good therapeutic option in cases of enhanced fibrinolysis. Such a therapy has to be guided by clinical judgment, TEG/ROTEM signs of lysis and “evident” bleeding, and not administered for routine prophylaxis [102] due to potential (albeit not demonstrated in a comprehensive meta-analysis) thrombotic risk [78, 147].
Except for VET, no commercial test evaluates global fibrinolysis. Measurement of individual components of the fibrinolytic pathway is unlikely to help in assessing and managing bleeding risk of cirrhosis [148].
The timing and severity of fibrinolysis is relevant to decide whether to treat or not this complication. It is not unusual to see a spontaneous resolution of lesser degree of fibrinolysis in the post-reperfusion phase. In the liver transplant settings, hyperfibrinolysis may occur in up to 60 % of the cases, but it is self-limiting in one third of these cases [101]. Hyperfibrinolysis typically subsides within an hour but may persist with poorly functioning or marginal grafts. This scenario rarely occurs in acute liver failure due to a high PAI-1 [149].
The drugs usually administered during liver transplant to treat hyperfibrinolysis are:
Aprotinin: associated with reduced blood loss and transfusion requirements. It is no longer available (withdrawn from the market for safety concerns) [146, 149].
Lysine analogues (tranexamic acid (TA) and E-aminocaproic acid (EACA)). TA and EACA are associated with lower risk of death when compared to aprotinin. Overall, TA seems to be the agent of choice in liver transplantation, being equally efficacious as the currently unavailable aprotinin [150]. According to a recent Cochrane review, antifibrinolytic therapy helps reduce blood loss and perioperative allogeneic blood transfusion [146].
As opposed to a blind prophylaxis with antifibrinolytics in liver transplantation, a goal-directed therapy, using thrombelastometry/graphy to assess fibrinolysis and evaluating the response to antifibrinolytic therapy, has been suggested [102]. Optimal timing and dose of TA during OLT have not been established [151]. Currently, in many centers, tranexamic acid is usually given to treat ongoing bleeding in 1–2 g increments (20–25 mg/kg body weight bolus) [102]; a continuous infusion of 1–2 mg/kg/h is also used in some institutions.
10.10.1.1 TEG-Based Indications
Fibrinolysis is defined as present when whole blood clot lysis index is less than 80 % [152]. Manufacturer reference values define fibrinolysis when LY30 min is >8 % and LY60 min >15 %. Therefore, the antifibrinolytic therapy is suggested by TEG monitoring and by the presence of coexisting microvascular oozing in the surgical field.
10.10.1.2 ROTEM-Based Indication
The presence of clinically relevant fibrinolysis can be detected as increased maximum lysis (ML >15 % of MCF), early starting reduction in MCF (<45 min), and improved measurement values obtained from a test containing aprotinin (APTEM) [150].
If APTEM clotting time (CT) is shorter than EXTEM CT, or CLI60 in EXTEM is <85 % during preanhepatic or early anhepatic, a therapeutic administration of tranexamic acid may be suggested.
If fibrinolysis is observed during the late anhepatic phase (CLI30EXTEM) <50 % therapeutic administration of tranexamic acid (25 mg/kg) is a useful option to reduce blood loss. Tranexamic acid administration is indicated after reperfusion when CLIEXTEM <50 % is present and persistent bleeding occurs [101].
10.10.2 Recombinant Activated Factor VII (rFVIIa)
Recombinant factor VIIa (rFVIIa) is recombinant human coagulation factor initially developed for bleeding episodes in hemophilia A and B patients who developed inhibitors against standard-factor replacements. It promotes hemostasis by activating the extrinsic pathway of the coagulation cascade. rFVIIa, bypassing factors VIII and IX, initiates coagulation of blood. The FVIIa tissue factor complex catalyzes the conversion of factor IX and factor X into the active proteases, leading to thrombin generation.
The administration of this drug during liver transplantation, enthusiastically welcomed after the first reports, and associated with reduction of blood loss and transfusion needs, was quickly stopped after two multicenter studies that showed an increased number of thromboembolic-related events and no reduction in blood loss and transfusion requirements [84, 153, 154].
According to a recent retrospective review, intraoperative versus preemptive administration of rFVIIa during liver transplant was associated with higher blood product use, lower graft and patient survival rates, longer ICU stays, and higher overall costs compared with preemptive administration [155]. The implication for clinical practice was the absence of clear evidence to promote FVIIa use in OLT apart from rescue therapy for uncontrolled bleeding after having ruled out other possible alternative causes and interventions [102].
Even if more trials are needed to adequately evaluate the use of prophylactic rFVIIa in liver transplantation, room for a specific role does not seem to be likely: as commented on by Yank and associates, however, available evidence is of low strength and too limited to compare the harms and benefits of rFVIIa in OLT [156].
10.10.3 Desmopressin
Desmopressin, a derivative of the antidiuretic hormone, was used for the first time to treat patients with hemophilia A and von Willebrand disease (vWD). Its indications were quickly expanded to bleeding disorders not involving a deficiency or dysfunction of factor VIII or vWf, as it can happen in liver diseases. Despite a reduction in the bleeding time in cirrhotic patients, controlled clinical trials were not able to confirm a positive effect in the management of acute variceal bleeding in ESLD patients [157]. Arshad and associates [158] showed that desmopressin administration had minimal hemostatic effects in cirrhotic patients while could be more effective in patients with mild disease: further clinical studies are required to confirm this point.
Conclusions
In the last 10 years, a sort of copernican revolution changed the interpretation of the hemostatic and coagulation profiles of the ESLD patients. Cirrhotic patients are not by definition auto-anticoagulated and then by default predisposed to bleeding: instead, they react, according to the different stimuli (e.g., endotoxemia) with a “rebalanced coagulation.” It is now clear that cirrhotic patients might be considered in some settings at greater risk of thrombosis than bleeding, even if routine plasmatic coagulation tests suggest hypocoagulability: this is the reason to explain the negative effects often associated with “fixed” prophylactic correction of laboratory values (mainly INR and R) with blood products. According to the above concepts, the use of rapid and more dynamic tests, as the viscoelastic TEG and ROTEM, are on the rise, providing a rapid diagnostic (and now reliable) bedside tests to aid the clinician in directing therapeutic interventions. An easy to use, rapid, flexible, and reliable VET point of care should guide a “step-by-step” evaluation of the ongoing hemostatic phenomenon/bleeding event, allowing an in vitro simulation of the correction (if and when needed) and the timely and appropriate correction of the disorder(s). In the near future, endothelial cells seeded onto microbeads or microchips flow chamber technique will improve the in vitro coagulation assessment, adding to the available technology the two missing links, endothelium and flow rate [144]. Algorithms, in some cases, even quite complex, are now available: however a strong evidence of their effects on outcome, blood loss, and transfusion needs is still lacking, particularly according to the most recent available experiences [144]. As a matter of fact, still unmet needs are consensus on MBT definition and shared attitudes/consensus on each step of the algorithm designed to guide coagulation monitoring and substitution/transfusion management. While VET point-of-care devices are not under discussion as monitors, cutoff values of the single TEG/ROTEM parameters are at least as far as we are aware. Thus, we consider wise and mandatory to design large clinical trials to find a consensus on values below which fibrinogen or PCC is to be administered (the same is for tranexamic acid in hyperfibrinolysis) or to be considered for an appropriate correction: the actual ever-growing economic constraints and an appropriate use of resources mandate this step.
Finally yet importantly, in the absence of a clinically relevant bleeding, pathological laboratory results (VET tests or conventional laboratory tests) are not an indication for a hemostatic intervention: correction of bleeding and, perhaps before, of pH, calcium, and body temperature are the first targets of the anesthesiologist: “cosmetic correction” of numbers is indeed beyond the aim of this chapter.
References
1.
Lisman T, Leebeek FW, de Groot PG (2002) Haemostatic abnormalities in patients with liver disease. J Hepatol 37:280–287PubMed
2.
Rapaport SI (2000) Coagulation problems in liver disease. Blood Coagul Fibrinolysis 11(Suppl 1):S69–S74PubMed
3.
Tripodi A, Salerno F, Chantarangkul V et al (2005) Evidence of normal thrombin generation in cirrhosis despite abnormal conventional coagulation tests. Hepatology 41:553–558PubMed
4.
Lu SN, Wang JH, Liu SL et al (2006) Thrombocytopenia as a surrogate for cirrhosis and a marker for the identification of patients at high-risk for hepatocellular carcinoma. Cancer 107:2212–2222PubMed
5.
Qamar AA, Grace ND, Groszmann RJ et al (2009) Incidence, prevalence, and clinical significance of abnormal hematologic indices in compensated cirrhosis. Clin Gastroenterol Hepatol 7:689–695PubMedPubMedCentral
6.
Ishikawa T, Ichida T, Matsuda Y et al (1998) Reduced expression of thrombopoietin is involved in thrombocytopenia in human and rat liver cirrhosis. J Gastroenterol Hepatol 13:907–913PubMed
7.
Peck-Radosavljevic M, Wichlas M, Zacherl J et al (2000) Thrombopoietin induces rapid resolution of thrombocytopenia after orthotopic liver transplantation through increased platelet production. Blood 95:795–801PubMed
8.
Aster RH (1966) Pooling of platelets in the spleen: role in the pathogenesis of “hypersplenic” thrombocytopenia. J Clin Invest 45:645–657PubMedPubMedCentral
9.
Wang CS, Yao WJ, Wang ST, Chang TT, Chou P (2004) Strong association of hepatitis C virus (HCV) infection and thrombocytopenia: implications from a survey of a community with hyperendemic HCV infection. Clin Infect Dis 39:790–796PubMed
10.
Garcia-Suarez J, Burgaleta C, Hernanz N, Albarran F, Tobaruela P, Alvarez-Mon M (2000) HCV-associated thrombocytopenia: clinical characteristics and platelet response after recombinant alpha2b-interferon therapy. Br J Haematol 110:98–103PubMed
11.
Bleibel W, Caldwell SH, Curry MP, Northup PG (2013) Peripheral platelet count correlates with liver atrophy and predicts long-term mortality on the liver transplant waiting list. Transpl Int 26:435–442PubMed
12.
Ordinas A, Escolar G, Cirera I et al (1996) Existence of a platelet-adhesion defect in patients with cirrhosis independent of hematocrit: studies under flow conditions. Hepatology 24:1137–1142PubMed
13.
Lisman T, Bongers TN, Adelmeijer J et al (2006) Elevated levels of von Willebrand Factor in cirrhosis support platelet adhesion despite reduced functional capacity. Hepatology 44:53–61PubMed
14.
Hollestelle MJ, Geertzen HG, Straatsburg IH, van Gulik TM, van Mourik JA (2004) Factor VIII expression in liver disease. Thromb Haemost 91:267–275PubMed
15.
Violi F, Leo R, Vezza E, Basili S, Cordova C, Balsano F (1994) Bleeding time in patients with cirrhosis: relation with degree of liver failure and clotting abnormalities. C.A.L.C. Group. Coagulation Abnormalities in Cirrhosis Study Group. J Hepatol 20:531–536PubMed
16.
Amitrano L, Guardascione MA, Brancaccio V, Balzano A (2002) Coagulation disorders in liver disease. Semin Liver Dis 22:83–96PubMed
17.
Lisman T, Leebeek FW, Mosnier LO et al (2001) Thrombin-activatable fibrinolysis inhibitor deficiency in cirrhosis is not associated with increased plasma fibrinolysis. Gastroenterology 121:131–139PubMed
18.
Huber K, Kirchheimer JC, Korninger C, Binder BR (1991) Hepatic synthesis and clearance of components of the fibrinolytic system in healthy volunteers and in patients with different stages of liver cirrhosis. Thromb Res 62:491–500PubMed
19.
vanDeWater L, Carr JM, Aronson D, McDonagh J (1986) Analysis of elevated fibrin(ogen) degradation product levels in patients with liver disease. Blood 67:1468–1473PubMed
20.
Szczepanski M, Habior A, Szczepanik A, Soszka A, Grel K (1994) Thrombin clotting time and fibrin polymerization in liver cirrhosis. Mater Med Pol 26:87–90PubMed
21.
Green G, Thomson JM, Dymock IW, Poller L (1976) Abnormal fibrin polymerization in liver disease. Br J Haematol 34:427–439PubMed
22.
Rijken DC, Kock EL, Guimaraes AH et al (2012) Evidence for an enhanced fibrinolytic capacity in cirrhosis as measured with two different global fibrinolysis tests. J Thromb Haemost 10:2116–2122PubMed
23.
Mammen EF (1994) Coagulopathies of liver disease. Clin Lab Med 14:769–780PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






