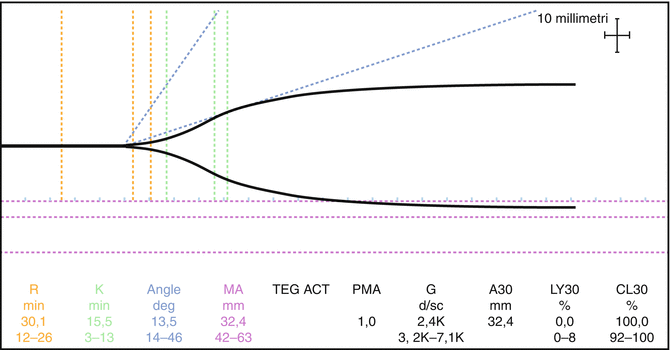
Fig. 14.1
ROTEM thromboelastometry profile performed before, during, and after the surgical intervention and then daily for 1 week. Reference values: INTEM CT 100–240 s, CFT 30–110 s, MCF 50–72 mm, EXTEM CT 38–79 s, CFT 34–159 s, MCF 50–72 mm, FIBTEM MCF 9–25 mm. (a) Before surgery: severe hypocoagulable profile characterized by a prolongation of CT and CFT in INTEM and EXTEM and severe reduction of MCF amplitude in INTEM, EXTEM, and FIBTEM. (b) Amelioration of hypocoagulable profile during neurosurgery after replacement therapy (FFP, platelets, and fibrinogen concentrates, PCC, tranexamic acid): stable prolongation of CT in INTEM, CFT in INTEM and EXTEM, and mild reduction of MCF amplitude in INTEM, EXTEM, and FIBTEM. (c) ROTEM profile 1 day after surgery before replacement therapy (FFP, platelets, and fibrinogen concentrates, PCC, tranexamic acid): prolongation of CT in INTEM and of CFT in INTEM and EXTEM and reduction of MCF amplitude in INTEM, EXTEM, and FIBTEM. (d) Stable hypocoagulable ROTEM profile on day 4 after neurosurgery: normalization of the activation phase (CT in INTEM and EXTEM) and less prolongation of the propagation phase (CFT in INTEM and EXTEM) associated with a lower reduction of MCF amplitude in FIBTEM. CT, clotting time; CFT, clot formation time; MCF, maximum clot firmness; FFP, fresh frozen plasma; PCC, prothrombin complex concentrates
After resection of tumor, an accurate hemostasis was pursued. Coagulation tests were repeated the day after the surgery (day 1), and they remained hypocoagulable (INR 1.50, aPTT 26 s, fibrinogen 1.10 g/L, platelet count 13 × 109/L). The corresponding ROTEM® profile is shown in Fig. 14.1c. In particular, a prolongation of CT and CFT in INTEM (298 s and 850 s, respectively), a normal CT and a prolonged CFT in EXTEM (53 s and 705 s, respectively), and a stable reduction of MCF amplitude in INTEM (23 mm) and EXTEM (26 mm) were demonstrated. MCF in FIBTEM (7 mm) was higher compared to basal MCF before surgery (3 mm). We decided to administer platelet concentrates, FFP 10 ml/kg, fibrinogen concentrates 2 g, PCC 20 U/kg, and tranexamic acid 1000 mg bid.
The coagulation monitoring was consecutively repeated daily after surgery for 7 days (days 2–7). ROTEM® (Fig. 14.1d) showed normalization of the activation phase (CT in INTEM and EXTEM) and less prolongation of the coagulation phase propagation (CFT in INTEM and EXTEM) associated with a lower reduction of MCF amplitude (6 mm in FIBTEM). Based on these findings, we decided to daily administer platelet concentrates, FFP 10 ml/kg, and fibrinogen concentrates 2 g until day 7 after surgery. No hemorrhagic episodes were observed during the following days. Two CT scans performed 7 and 30 days after surgery ruled out a cerebral hematoma occurrence.
14.3.1 Comment
Prompt availability of coagulation test results during surgery is mandatory for appropriate bleeding management, in particular for patients with severe bleeding for whom delays in the treatment must be minimized. The current solution to avoid unacceptable turnaround times, as recommended in European guidelines, is to implement viscoelastic devices to improve the management of hemostatic therapy.
In our patient, platelets concentrates, FFP, and PCC were given in order to ameliorate the severe coagulopathy detected by ROTEM® assay. In addition to platelets defect, decreased synthesis of clotting factors and inhibitors, and hyperfibrinolysis, it is worth noting that coagulopathy-related bleeding in liver cirrhosis is also due to an abnormal fibrinogen synthesis/polymerization. This acquired dysfibrinogenemia is characterized by an increase in sialic acid content leading to an impaired fibrin monomer polymerization. It can be functionally detected by a prolongation of the thrombin time, which correlates with the increase in fibrinogen sialic acid content. In our case, ROTEM analysis was useful to demonstrate the presence of a dysfunctional fibrinogen through the measurement of MCF in FIBTEM, which showed a reduced fibrin clot formation despite plasma levels of fibrinogen measured by Clauss assay which were greater than 1 g/L and thus enough for hemostasis. Importantly, since FIBTEM is designed to measure elasticity of the fibrin clot under platelet inhibition by cytochalasin D, FIBTEM MCF should not be considered as a measurement of fibrinogen concentration, but mainly a measurement of mechanical properties of the fibrin clot which are only in part related to fibrinogen concentration. Low FIBTEM MCF may be interpreted as an indication for fibrinogen deficiency or fibrin polymerization disorders. Our findings confirm that the viscoelastic measure of the fibrinogen allows the evaluation of functional fibrinogen polymerization without platelet “noise” and may be a marker of a reduced fibrin polymerization and a bleeding tendency. These findings guided replacement therapy by fibrinogen concentrates in our patient and the use of antifibrinolytic agents to stabilize fibrin clot.
Fibrinogen plays an important role in the coagulation process and clot stabilization binding of FXIII. In addition, it plays a central role in platelet activation and aggregation by binding to the platelet glycoprotein receptor GPIIb/IIIa. Interestingly, in experimental studies it has been shown that fibrinogen administration counteracts the effects of low platelet count and improves platelet aggregation. In our thromboelastometry whole blood analysis, the decrease in platelets could be offset by an increase in fibrinogen/fibrin polymerization, which results in the restoration of MCF in FIBTEM compared to baseline levels following administration of fibrinogen concentrate. It seems that the amelioration of fibrinogen concentration would improve clot formation and platelets aggregation and allows for a safe surgery despite the low platelets count.
Our experience suggests that a ROTEM®-based approach could be helpful in identifying the blood components necessary to restore coagulation. In fact, no intracranial bleeding complications occurred either during the intervention or in the whole postoperative course. Thromboelastometry is able to optimize the choice of replacement therapy with hemostatic products, thus preventing hemorrhagic complications. Another considerable advantage is the short time required to use the algorithm approach, which makes it possible to obtain a rapid and focused intervention. Larger and prospective studies are needed to validate the use of ROTEM “theragnostic” approach in the management of neurosurgery or invasive procedures in coagulopathic population.
14.4 Case 3: Postoperative Bleeding Management in a Liver Transplant Patient
A 63-year-old male underwent liver transplantation for HBV-related cirrhosis. The preoperative laboratory profile is reported below:
Hemoglobin, 7.9 g/dL; hematocrit, 24 %; platelet count, 94 × 106/L
Bilirubin, 2.85 mg/dL; creatinine, 2.14 mg/dL; albumin, 3.2 g/dL
PT, 22 %; INR, 2.98; aPTT, 1.82; fibrinogen, 119 mg/dL
During surgery, the following blood products and substitutes were administered: red blood cells (RBCs), 3876 mL; platelet concentrate, 1 U; and fibrinogen concentrate (Haemocomplettan® P, CSL Behring, Marburg, Germany), 4 g. Additionally, 1 g of tranexamic acid was administered.
Post-surgery, a native thromboelastographic (TEG) test was done, without showing significant alterations (Fig. 14.2). The corresponding standard laboratory data are reported below:
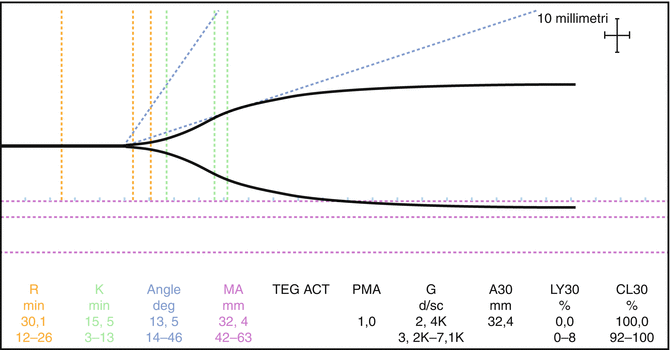

Fig. 14.2
1st postoperative TEG
Hemoglobin, 10.1 g/dL; hematocrit, 29.2 %; platelet count, 52 × 106/L
Bilirubin, 4.36 mg/dL; creatinine, 1.84 mg/dL; albumin, 1.2 g/dL
PT, 16 %; INR, 3.96; aPTT, 3.64; fibrinogen, 109 mg/dL
Two hours after extubation, a moderate bleeding from the abdominal drains starts, without hemodynamic compromise. After one hour of ongoing bleeding, a moderate hypotension appears (systemic blood pressure 95/50 mmHg, heart rate 100 beats/min). Lactated Ringer’s solution (500 mL) and 2 units of fresh frozen plasma (FFP) were infused. The surgeon in charge is alerted but did not judge that the patient merits further surgery. A second TEG test is performed, highlighting a worsened coagulation status (Fig. 14.3). The blood loss from the drains became more evident in the subsequent hours, and the patient developed signs of shock. The circulating volume is replaced with an additional 2 units of RBCs, 1100 mL of FFP, 500 mL crystalloid, and 500 mL colloid fluids for a total of 2 l of hemo-derivatives and 1 l of colloids/crystalloids. A blood sample is taken for laboratory tests and TEG analysis. TEG with heparinase is severely impaired (Fig. 14.4). The laboratory data are as follows: