TOPICS
2. Definitions and Taxonomy of Postpartum and Major Obstetric Hemorrhages
3. Epidemiology and Risk Factors
4. Management of Planned and Unexpected Obstetric Hemorrhage
INTRODUCTION
Postpartum hemorrhage (PPH) remains a leading cause of maternal morbidity and mortality worldwide. Epidemiologic studies show an increasing frequency and severity of PPH in the past decade, due to an increase in uterine atony and placenta accreta, percreta, and increta. Management of PPH requires prompt and efficient multidisciplinary intervention to improve uterine tone; provide adequate fluid and hemodynamic resuscitation, administer blood products, and decide whether additional procedures such as interventional radiology or surgery are needed. Optimal management of PPH requires adequate communication between all team players (nurses, midwives, obstetricians, anesthesiologists, hematologists/blood bank, surgeons, interventional radiologists, and intensive care unit staff). Teamwork is facilitated when protocols are in place to allow human allocation and tasks to be well defined along with adequate record keeping. Algorithms for the use of blood products, fibrinogen, recombinant activated factor VII (rFVIIa), tranexamic acid, blood cell salvage devices, and other conservative maneuvers (Bakri balloon, B-Lynch procedure, interventional radiology) should be in place to reduce the need for hysterectomy and massive transfusion. Adequate documentation, debriefing after clinical care, and audits should facilitate monitoring of the success of such protocols. Practice guidelines and recommendations established by national societies and organizations have flourished in the literature over the past 5 to 8 years to assist clinicians in the prevention and management of PPH, of which the practice bulletin of the American College of Obstetrics and Gynecology (ACOG) in 2006,1 the International Confederation of Midwives, the International Federation of Gynecology and Obstetrics (FIGO) initiative in 2006,2 the clinical practice guideline of the Society of Obstetricians and Gynaecologists of Canada (SOGC) in 2009,3 the California Maternal Quality Care Collaborative (CMQCC) guide in 2010,4 the Royal College of Obstetricians and Gynaecologists (RCOG) guidelines in 2011,5 and the most updated World Health Organization (WHO) recommendations in 20126 are just a few examples.
The goal of this chapter is to present a practical and updated overview on current modalities and recommendations that are available, to improve the management of planned and unplanned obstetric hemorrhages, and to prevent fatal outcomes for both mothers and their infants.
DEFINITIONS AND TAXONOMY OF POSTPARTUM AND MAJOR OBSTETRIC HEMORRHAGES
The clinical estimation of blood loss during delivery is frequently inaccurate, in general underestimated, and the presence of amniotic fluid may be a confounding factor. It is important to bear in mind that healthy women will not become symptomatic before a significant amount of blood loss has occurred. In other words, by the time significant obstetric hemorrhage has been observed, women may already have lost 10% to 15% of their circulating blood volume. Nonetheless, whereas symptoms, hemodynamic parameters, a hematocrit value or the need for blood products would appear valuable to determine whether a hemorrhage is significant or not, the diagnosis is usually based solely on the estimated blood loss (Table 20-1).
Table 20-1. Definitions and Taxonomy of Postpartum Hemorrhage (PPH) and Major Obstetric Hemorrhage (MOH)
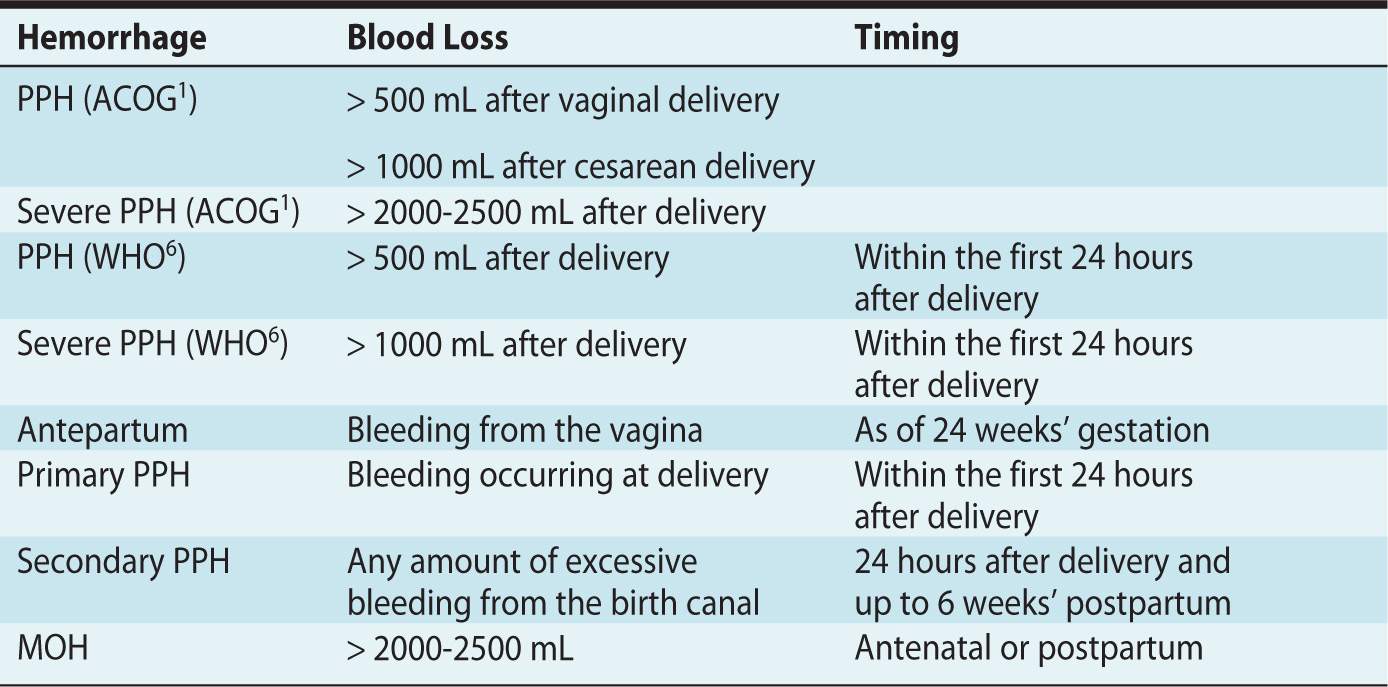
• PPH is defined by ACOG as blood loss exceeding 500 mL after a vaginal delivery or 1000 mL after a cesarean delivery.1 The WHO has defined PPH as a blood loss greater or equal to 500 mL within 24 hours after birth regardless of mode of delivery, and severe PPH is a blood loss greater or equal to 1000 mL in the first 24 hours postpartum.6
• Severe PPH is considered when blood loss is deemed to be greater than 2000 to 2500 mL.
• Antepartum hemorrhage is defined as bleeding, usually but not always manifested from the vagina after 24 weeks’ gestation, and is associated with placenta previa, placental abruption, and uterine rupture or trauma.
• Primary PPH is defined as a bleeding that occurs before delivery (antepartum) and within the first 24 hours’ postpartum.
• Secondary PPH is considered with any abnormal or excessive bleeding from the birth canal occurring between 24 hours’ and up to 6 weeks’ postpartum. The most common etiology of secondary PPH is retained placental tissue.
• Major obstetric hemorrhage (MOH) is the term typically used in the United Kingdom and Europe to describe major antenatal or postpartum hemorrhage. There is no consensus on what constitutes a major blood loss; blood loss exceeding 1500 mL, a hemoglobin decrease of more than 4 g/dL, or an acute transfusion requirement of more than 4 units of red blood cells are suggested criteria.
EPIDEMIOLOGY AND RISK FACTORS
The incidence of PPH has been steadily increasing in the last decade in the United States7 and represents the second leading cause of maternal mortality after cardiovascular disorders. PPH complicates 2.9% of deliveries, is the second leading cause of intensive care unit admission among pregnant women and is associated with close to 20% of all in hospital maternal deaths after delivery in the United States. Atonic PPH is currently the leading cause of PPH irrespective of mode of delivery and accounts for up to 79% of all PPH.
The etiologies of PPH can be classified as follows (four Ts):
1. Tone: abnormal uterine contractility or any condition that increases uterine distention, uterine muscle fatigue, uterine distortion or abnormality, chorioamnionitis, or pharmacologically induced uterine relaxation
2. Tissue: retained placental products or the morbidly adherent placenta that results in the abnormal placental detachment (eg, placenta accreta, percreta, increta) in the third stage of labor
3. Trauma: genital tract injury (laceration) or uterine injury (uterine rupture or inversion)
4. Thrombin: preexisting (eg, hemophilia, von Willebrand’s disease [VWD]) or acquired abnormalities of coagulation (severe preeclampsia; hemolysis, elevated liver enzymes, low platelets [HELLP]; disseminated intravascular coagulation [DIC]) or anticoagulant therapies
A thorough antenatal screening may help identify risk factors for PPH and allow for some preparation if PPH is expected. In particular, identifying the risk for placenta accreta in the event of a repeat cesarean delivery with a placenta previa is critical. Other antenatal factors that substantially increase the risk for PPH include:
• Suspected or proven placental abruption
• Known placenta previa
• Multiple pregnancy
• Hypertensive disorders of pregnancy (preeclampsia)
Uterine Atony
Uterine atony is characterized by failure of the myometrium to contract after delivery, associated with excessive bleeding from the noncontracting placental implantation site. The rising rates of PPH in the last decades have been driven by the increasing incidence of atonic PPH, although specific explanations for these temporal trends are still under investigation.8
Unlike other causes of obstetric hemorrhage such as placental abnormalities that may be detected through antenatal screening, uterine atony may be difficult to predict, although risk factors for its occurrence after a vaginal delivery have been identified.
Demographic factors associated with uterine atony include:
• Advanced maternal age
• Obesity
• Ethnicity (Hispanic, Asian/Pacific Islander)
Obstetric factors associated with uterine atony include:
• Uterine overdistention (polyhydramnios, multiple gestation, macrosomia)
• Labor induction and prolonged exposure to oxytocin
• Prolonged labor and protracted second stage
• Chorioamnionitis
• Preeclampsia
• Previous PPH
• Pharmacologic agents relaxing the uterus (tocolytics, MgSO4, halogenated anesthetics)
Active management of the third stage of labor is essential to facilitate placental separation and delivery, and promote contraction of the uterus to shorten the third stage of labor, prevent uterine atony and excessive bleeding. The duration of the third stage has been shown to correlate with the risk for PPH; a third stage of labor longer than 18 minutes is associated with a significant risk of PPH and the odds for PPH are 6 times higher if the third stage is longer than 30 minutes. The three key interventions of active management are presumed to be:
• Use of a prophylactic uterotonic agent (oxytocin at birth)9
• Early umbilical cord clamping, although evidence has emerged that it is not necessarily beneficial and no longer recommended2
• Controlled umbilical cord traction, although this has also been recently refuted
Pharmacologic management of uterine atony is essential, and oxytocin alone or in combination with other uterotonic agents is recommended based on the clinical situation and presence of contraindications to administrate second-line agents.
Retained Placental Products
Retained placental products (or products of conception) occurs in 0.1% to 3.3% of vaginal deliveries and is the second leading cause of PPH usually associated with uterine atony. Etiologies for a retained placenta include failure of the myometrium behind the placenta to contract resulting from an adherent placenta, a detached placenta trapped behind a closed cervix, or a placenta accreta. Given the entirely different etiologies and underlying pathologies, management should be based on the suspected diagnosis, which can be established with ultrasound imaging.
Sublingual or intravenous nitroglycerin along with controlled traction of the umbilical cord has been evaluated as an effective way to facilitate placental separation and removal, with mixed results. Studies evaluating the intraumbilical injection of oxytocin or other uterotonics also have yielded mixed results and do not seem to reduce the need for a manual removal of retained products.
Finally, anesthesia for manual removal of a retained placenta, requires a dense anesthetic block, via an indwelling epidural catheter or a de novo single shot spinal, with a T6 level to ensure maternal comfort and possibly the need for a uterine relaxant if the lower uterine segment is obstructing.10
Placental Abruption
Placental abruption is characterized by the premature separation of the placenta, which can be complete or partial, and is a serious obstetric complication that occurs in more than 1% of pregnancies. Placental abruption results in more than 50% preterm delivery. Overall perinatal mortality with placental abruption is high (119 in 1000 births), with 7% attributed to the abruption itself and a majority of deaths occurring in utero (77%). The etiology of placental abruption remains unclear but is thought to be the consequence of abnormal trophoblast invasion leading to the rupture of the spiral arteries causing premature separation of the placenta. Ultrasound imaging may show retroplacental clot(s) or bleeding but does not seem to predict the extent of placental abruption, and the diagnosis of abruption should be based on clinical criteria, which include vaginal bleeding with a nonreassuring fetal heart rate with or without tachysystole (uterine hypertonus), abdominal tenderness and pain.
More than 50 different risk factors or markers for placental abruption have been reported, with smoking (maternal and paternal), preeclampsia, and the history of a previous placental abruption being the strongest. Others include maternal age, chronic hypertension, cocaine use, hyperhomocysteinemia, inherited or acquired thrombophilia, abdominal trauma, premature rupture or membranes, and chorioamnionitis.
Placental abruption, particularly in the context of preeclampsia, can result in DIC, hemolysis, acute renal failure, and pulmonary edema, and serious maternal complications are not infrequent. Anesthetic management for an emergent cesarean delivery will depend on the urgency of the situation (fetal and maternal) and the maternal hemodynamic and coagulation status.
Placenta Previa
Placental previa occurs when there is an abnormally low attachment of the placenta, partially or totally covering the cervical os. Further distinctions can be made with regards to the distance of the placenta to the cervical os, as follows:
• Total previa: completely covering the cervical os
• Partial placenta previa: partially covering the cervical os
• Marginal placenta previa: close but not covering the cervical os
• Low-lying placenta: placental edge in close proximity to the cervical os (of note, 90% of placentas identified as low-lying in early pregnancy will resolve by the third trimester)
The incidence of placenta previa is 0.5%, but increases occur with multiparity, prior spontaneous and induced abortions, and smoking, as well as in Asian women.11 Additional independent risk factors for placenta previa are prior cesarean delivery, advanced maternal age, and infertility treatments. The current rising trends for each of these factors contribute to the increasing incidence of placenta previa. In comparison with risk factors for placental abruption, placenta previa is associated with conditions that precede pregnancy, whereas placental abruption is more likely to be affected by conditions occurring during pregnancy.
Delivery options should be based on the distance between the lower edge of the placenta and the internal cervical os and, in the absence of antepartum bleeding, a cesarean delivery at term may be scheduled.
The anesthetic technique for a primary cesarean delivery need not be different from that otherwise planned for cesarean delivery in general (spinal or combined spinal-epidural anesthesia), with the exception that the potential blood loss may be greater, 1000 to 1500 mL, blood products should be available and adequate venous access ensured. Autologous blood salvage (cell saver) may be considered, particularly if the patient has a rare blood type or is refusing blood products (Jehovah’s witness).
Other important considerations with a placenta previa are:
• Up to 3% of placenta previa are associated with a placenta accreta even in the absence of a previous cesarean delivery (or uterine scar). Therefore, specific ultrasound screening (and possibly magnetic resonance imaging [MRI]; see below) is essential.
• If a placenta accreta is suspected (with or without a previous cesarean delivery), a thorough multidisciplinary care plan is needed for the cesarean, including the possibility of hysterectomy (see under Placenta Accreta).
Placenta Accreta
Abnormal placentation can be life threatening because of the risk of massive hemorrhage at the time of delivery. In addition, maternal morbidity is significant because of the probable need for peripartum hysterectomy, transfusion of blood and blood products, damage to surrounding organs, and prolonged hospitalization, including intensive care unit admission. Clinically, placenta accreta becomes problematic during delivery when the placenta does not completely separate from the uterus and is followed by massive hemorrhage (typically 3-5 L of blood loss). The following definitions may be used:
• Placenta accreta is defined by the absence of a decidua basalis and is used to describe the clinical condition when part of the placenta, or the entire placenta, has invaded and is inseparable from the uterine wall.
• Placenta increta describes the situation when the chorionic villi invade into the myometrium.
• Placenta percreta describes the invasion through the myometrium and serosa, and possibility of adjacent organ invasion, such as the bladder.
The incidence of placenta accreta has been reported to be 1 in 533 pregnancies for the period 1982-2002,12 which is significantly higher than previously reported and is likely paralleling the increase in cesarean deliveries. Numerous epidemiologic studies have demonstrated an increased incidence of accreta in women with a subsequent placenta previa and women with a history of previous cesarean delivery (with or without a placenta previa overlying the uterine scar). Indeed, the estimated rate of placenta accreta in women with a placenta previa undergoing a fourth or higher order repeat cesarean delivery is at least 60%. A recent study demonstrated that a primary elective cesarean delivery prior to labor is more likely to preserve fertility and result in subsequent pregnancy in women with placenta previa as compared with an urgent cesarean delivery performed when the parturient is in labor.13 This study, suggesting that absence of labor at the time of the primary cesarean delivery, or in other words, that the uterine incision occurs in a thick quiescent myometrium, may modify the risk of future possibility of placenta accreta in subsequent pregnancies merits further investigation.
The recent ACOG Committee opinion on placenta accreta14 emphasizes the following:
• The diagnosis of placenta accreta is usually established by prenatal ultrasonography and occasionally supplemented by MRI. In a study with sonographic and MRI findings that were each verified with the final diagnosis at delivery, ultrasonography had a sensitivity of 93% and specificity of 73%, while that of MRI was 80% and 65%, respectively (which was not significantly different).15 A recent study to evaluate placental MRI in the diagnosis and surgical management of placenta accreta suggested that MRI may be more accurate than ultrasound to establish a precise topographic mapping of the invaded area, particularly in relation with the vascular anatomy and blood supply, which could be extremely useful to guide the surgical dissection during hysterectomy.16
• If there is a strong suspicion of an abnormal placental invasion, obstetricians and radiologists practicing at small hospitals or at institutions with insufficient blood bank supply or inadequate availability of subspecialty (hematologist, gynecologic oncology surgeons, urologists, intensivists, hematologists) and support personnel should consider patient transfer to a tertiary perinatal care center.
• The timing of delivery should be individualized. However, combined maternal and neonatal outcomes are optimized in stable patients with delivery at 34 weeks’ gestation (even without an amniocentesis to demonstrate fetal lung maturation). The goal is for a planned delivery as greater blood loss, and complications have been shown to occur in emergent cesarean hysterectomies versus planned cesarean hysterectomies.
• For the surgical approach, the recommended management of suspected placenta accreta is planned preterm cesarean hysterectomy with the placenta left in situ because removal of the placenta is associated with significant hemorrhagic morbidity. However, alternative approaches may be considered for women who have a strong desire to preserve fertility. Generally, planned attempts at manual placental removal should be avoided. If hysterectomy becomes necessary, the standard approach is to leave the placenta in situ, quickly use a “whip stitch” to close the hysterotomy incision, and proceed with hysterectomy.
• Blood loss in women with a placenta accreta can be expected to be massive. In fact, blood loss was shown to exceed 5000 mL in 42% of women with a known diagnosis of placenta accreta, although there is no reliable individual predictor of blood loss.17 Current recommendations for blood replacement are a 1:1:1 ratio of packed red blood cells (PRBCs) to fresh frozen plasma (FFP) and platelets, which should be available in the operating room from the start. Institutionally established massive transfusion protocols should be followed. Additional units of blood and coagulation factors should be infused quickly and as necessitated by the patient’s vital signs and hemodynamic stability. It is helpful to perform multidisciplinary drills to prepare for hemorrhage.
• Autologous blood salvage devices (cell saver) are safe and valuable in the management of a MOH.
• Current evidence for interventional radiologic procedures prior to the cesarean delivery is insufficient to make a firm recommendation about the use of balloon catheter occlusion or embolization to reduce blood loss and improve surgical outcome. However, specific situations may warrant their use.
• Methotrexate has been proposed as an adjunct treatment for placenta accreta. However, there is insufficient evidence to recommend its use routinely, and although uterine conservation was initially achieved in some instances, patients may subsequently develop PPH requiring hysterectomy.
There is no consensus regarding the choice of anesthetic to adopt for the management of a cesarean delivery in a woman with a suspected placenta accreta. Nowadays, most obstetric anesthesiologists would opt for a continuous neuraxial anesthetic (combined spinal-epidural, or continuous epidural or spinal) to allow titration and redosing of local anesthetics in the event of prolonged surgical time. This also offers the advantage of allowing the patient to be awake for the delivery of the infant, the partner to be present if clinical conditions permits, and avoids a general anesthetic for both the mother and infant; another advantage of neuraxial techniques is that they can be used to manage postcesarean/hysterectomy analgesia with the indwelling catheter. If needed due to massive hemorrhage or discomfort, conversion to general anesthesia could be provided at a later stage.
Trauma
ANTENATAL ABDOMINAL TRAUMA
Pregnant women admitted because of an abdominal trauma require appropriate monitoring and management. Approximately 25% of patients, preterm labor, placental abruption, uterine rupture, or other circumstances may require a prompt delivery.
LACERATIONS OF THE CERVIX, VAGINA, OR PERINEUM
Vaginal and cervical lacerations during a vaginal delivery tend to occur more commonly with a precipitous delivery, with macrosomia, shoulder dystocia, an instrumental delivery, and an episiotomy (mediolateral in particular). Risk factors for cervical laceration specifically are young maternal age, induction of labor, cerclage, and vacuum extraction. Asian women appear to be at greater risk for vaginal and significant perineal tears (third-degree and fourth-degree) after a vaginal delivery.
Uterine Inversion
A uterine inversion is an extremely rare event but one that can result in serious adversity. It is usually associated with prolonged labor, grand multiparity, a fundal placenta, and third-stage manipulation using excessive cord traction, during which the internal surface of the uterus may be forced partially or completely through the uterine cervix. The incidence of acute uterine inversion has substantially decreased with the introduction of active management of third stage of labor protocols. Management of uterine inversion has two crucial components: the immediate restoring of the uterus to its normal position and treatment of the hemorrhagic shock. Immediate uterine reversion will prevent massive blood loss and hemodynamic instability but is not always successful. Nitroglycerin has been used to provide relaxation and enable uterine reversion because the lower uterine segment is often a mechanical obstacle. Regional anesthesia does not provide uterine relaxation but may be helpful by providing analgesia. In the past, case reports suggested the use of general anesthesia with volatile agents in order to replace the uterus. In the most resistant inversions, a surgical correction may be required; laparoscopic reduction has been reported.18
Uterine Rupture or Dehiscence
Uterine rupture is defined as a full-thickness separation of the uterine wall and overlying serosa. It is associated with uterine bleeding; fetal bradycardia (and other abnormalities of fetal heart rate); and protrusion or expulsion of the fetus, placenta, or both into the abdominal cavity. In contrast, uterine scar dehiscence constitutes separation of a preexisting scar that does not disrupt the overlying visceral peritoneum.
The incidence of complete uterine rupture is extremely low, and the most important risk factors are a previous uterine scar due to previous cesarean delivery, history of dilation and curettage, uterine polypectomy, endometrectomy, or an intrauterine device. Other risk factors include multiple previous cesarean deliveries, a short interpregnancy interval, a vertical uterine incision, and a locked single-layer uterine closure. To avoid catastrophic maternal and neonatal outcomes, women with a vertical uterine scar (postcesarean delivery or myomectomy) should have a planned cesarean delivery at 37 weeks’ gestation.
The incidence of uterine rupture in women attempting a trial of labor after previous cesarean delivery (TOLAC) fluctuates and is estimated to range between 0.5% and 4%.19,20 There is no available predictive model to estimate the risk of uterine rupture in women selecting a TOLAC, and although prostaglandins for induction of labor are considered to increase the risk of uterine rupture, the evidence is relatively scarce.21 It has been suggested that the duration of labor, rather than the induction of labor per se, is the factor associated with the risk of rupture. A recent Cochrane review concluded that there is insufficient data to make any recommendations with regards to the optimal method of induction of labor in women with a previous cesarean delivery.
Uterine rupture is typically characterized by (1) a sudden constant abdominal pain that is recognizable because it persists in between contractions (although cessation of contractions can also occur), (2) a nonreassuring fetal heart rate tracing, and (3) antepartum bleeding (which may remain concealed). Pain is not usually masked by current regimens of low-dose epidural labor analgesia (ie, dilute concentrations of local anesthetics); however, frequent epidural dosing (top-ups for breakthrough pain) should raise suspicion for an impending uterine rupture22 in women having a TOLAC as well as referred shoulder pain.
In 2010, after joint agreement by ACOG and American Society of Anesthesiologists (ASA), the ACOG statement on TOLAC includes the following: “Because the risks associated with TOLAC and uterine rupture may be unpredictable, the immediate availability of appropriate facilities and personnel (including … a physician capable of monitoring labor and performing cesarean delivery, including an emergency cesarean delivery) is optimal.”19 Issues surrounding the requirement of “immediate availability” have emerged,23 because other emergent obstetric complications such as placental abruption or umbilical cord prolapse are equally catastrophic maternal and neonatal outcomes as uterine rupture, yet immediate availability is not mandated. ACOG supports the use of epidural for labor analgesia during TOLAC, because more women may opt for a trial of labor if they can benefit of adequate pain relief. As once believed, an epidural will neither mask the signs and symptoms nor delay the diagnosis of uterine rupture; changes in fetal heart rate and uterine patterns, and in particular bradycardia, are the most common signs of uterine rupture.
The ASA guideline recommendations published in 2007 state that “neuraxial techniques should be offered to patients attempting vaginal birth after previous cesarean delivery” and that “it is also appropriate to consider early placement of a neuraxial catheter that can be used later for labor analgesia or for anesthesia in the event of an operative delivery.”24
As emphasized in a recent review on the role of the anesthesiologist during TOLAC23:
• Anesthesiologists should be involved in the antenatal counseling of women planning a TOLAC.
• A preanesthetic evaluation early in labor should be enabled.
• Women may have limited clear liquids; however, nothing-by-mouth is recommended if they have a potentially difficult airway, a nonreassuring fetal tracing, or if they are in active labor.
• Early neuraxial labor analgesia should be encouraged.
• A well-functioning epidural catheter may be used for cesarean delivery and may reduce the need for a general anesthetic, even in the event of an urgent cesarean delivery (general anesthesia has been shown to increase the risk of PPH25).
Preexistent or Acquired Coagulopathy
Coagulopathies that increase the risk of PPH may be inherited (eg, hemophilia, VWD, Glanzmann thrombasthenia) or may be acquired during pregnancy (severe preeclampsia, HELLP syndrome) and at the time of delivery (DIC). In addition, women with thrombophilias or other indications for anticoagulation or antithrombotic medication during pregnancy may be at increased risk for PPH.
Pregnancy is associated with a progressive increase in the levels of several coagulation factors including fibrinogen; factors VII, VIII, X, and XII; and von Willebrand’s factor (VWF). The increase in these coagulation factors occurs gradually during pregnancy and accelerates in the third trimester. Factors II, V, IX, XI, and XIII levels are slightly elevated or unchanged during normal pregnancy. Similar changes may also be seen in women with inherited bleeding disorders, which may lead to normalization of the hemostatic defect in women with some bleeding disorders such as VWD or carriers of hemophilia A. In rare bleeding disorders, hemostatic abnormalities seem to persist throughout pregnancy, especially if the defect is severe.
When assessing the potential for bleeding in pregnant women with coagulopathies, a detailed bleeding history, family background, and obstetric history should be assessed, along with coagulation tests and factor levels. Having a severe bleeding disorder is not in itself an indication for a cesarean section delivery and, in most cases, normal vaginal delivery can be planned. In some cases, cesarean delivery may be deemed safer for obstetric indications. The management plan should be individualized, and a written multidisciplinary delivery plan should be established during the third trimester, one that is available to all of those involved in the patient’s care (including the woman herself). Good communication between hematologists, obstetricians, anesthesiologists, midwives, and neonatologists is essential for the safe management of labor and delivery.
Neuraxial labor analgesia or neuraxial anesthesia for cesarean delivery has often been unjustifiably denied to women with bleeding disorders because of the potential risk of spinal/epidural hematoma formation with subsequent spinal cord compression. Epidurals or spinals can be offered to women with normalized factor levels during pregnancy or who have normal factor levels as a result of treatment, but women should be thoroughly informed about the risks and benefits of neuraxial techniques for labor analgesia and the availability of alternative modalities (N2O and/or systemic opioids) as well as the risks of neuraxial anesthesia (spinals) for cesarean delivery. Factor levels also need to be maintained in the normal range at the time of epidural catheter removal. Neuraxial analgesia/anesthesia should not be used in situations in which hemostasis is not guaranteed, such as in patients with uncorrected severe deficiencies or in whom there is poor correlation between the bleeding risk and factor levels.
Several important points during management of labor and delivery include the following:
• If the fetus is at risk of having a bleeding disorder, invasive monitoring, such as fetal blood sampling, fetal scalp electrodes, and the use of vacuum extraction and midcavity forceps, should be avoided.
• Prolonged second stage of labor also increases the risk of neonatal hemorrhage. Therefore, in cases in which this is likely, an early recourse to cesarean delivery is suggested.
• Trauma to the maternal genital and perineal areas should be minimized during delivery.
Particular challenges will be faced by pregnant women with inherited coagulation factor deficiencies during the puerperium. Pregnancy care of women with such disorders should be managed by high-risk obstetric specialists, aided by a multidisciplinary team that includes a hematologist with expertise in hemostasis, anesthesia and, if appropriate, a pediatric hematologist, and the delivery should be planned in a hospital that has a hemophilia center. Factor levels should be checked antenatally, at 28 and 34 weeks’ gestation, and certainly before invasive procedures Frequent follow-up may be required if prophylactic factor treatment is given during pregnancy. All women with factor deficiencies should deliver in a unit that has ready access to a blood bank and pathology service with the required factor treatments and laboratory tests. Specific targeted management to compensate for the deficient factor(s) according to the bleeding disorder will be required (and is not discussed in this chapter).26,27
Finally, at the time of delivery, placental abruption, amniotic fluid embolism, and retained placental products may result in acute intravascular activation of coagulation, which causes thromboembolic complications and consumption coagulopathy, resulting in severe hemorrhage.28 The central underlying pathophysiologic perturbation in the coagulopathy associated with these syndromes is the release of tissue factor from the placenta and amniotic fluid into the circulation, in combination with low levels of physiologic anticoagulant factors during pregnancy. DIC is a catastrophic event, and rFVIIa has been suggested as an effective strategy,29 although evidence to guide hematologic management of DIC is lacking.
MANAGEMENT OF PLANNED AND UNEXPECTED OBSTETRIC HEMORRHAGE
Pharmacologic Management
UTEROTONICS AGENTS (TABLE 20-2)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








