TOPICS
INTRODUCTION
A variety of medications are used in obstetric practice by both obstetricians and anesthesiologists. These medications may differ from those used in the surgical operating room. This chapter reviews the medications commonly used in obstetrics.
TOCOLYTIC MEDICATIONS
Tocolytics are used by obstetricians to treat preterm labor in an attempt to prevent premature birth. Preterm birth is the leading cause of perinatal morbidity and mortality and complicates approximately 12% of pregnancies in the United States.1 Recent Cochrane meta-analyses of tocolytic agents determined that calcium channel blockers and oxytocin antagonists can delay delivery by 2 to 7 days,2 that β-mimetic drugs delay delivery by 48 hours but carry greater side effects,3 that there is insufficient evidence regarding cyclooxygenase (COX) inhibitors,4 and that magnesium sulfate is ineffective.5 Anesthesiologists may be more involved with other uses of these drugs, including in the treatment of uterine tetany, uterine inversion, retained placenta, and fetal head entrapment.
Magnesium Sulfate
INDICATIONS
Magnesium sulfate has been used for suppression of preterm labor and for seizure prophylaxis in patients with severe preeclampsia. However, it is no longer used as a tocolytic agent because it has not been shown to be more effective than placebo in preventing preterm labor and delivery.6 Although the primary indication for magnesium sulfate administration remains prevention of seizure activity in patients with severe preeclampsia, a newer use of magnesium administration during preterm labor involves benefit to the brain of the unborn fetus. Studies have demonstrated that premature infants as young as 23 weeks, born to mothers treated with magnesium sulfate, have improved developmental outcomes.7
MECHANISM OF ACTION
The systemic effects of magnesium sulfate administration are widespread and require the need for careful monitoring. Magnesium crosses the blood-brain barrier and decreases irritability of the central nervous system (CNS) and decrease N-methyl-D-aspartate activity. These effects likely account for the anticonvulsant and brain protection properties. Magnesium also competes with calcium for binding sites on the sarcoplasmic reticulum, reducing intracellular calcium levels and reducing the force and frequency of muscle contraction in both skeletal and smooth muscle.8 Magnesium decreases the presynaptic release of acetylcholine, thereby reducing activity at the neuromuscular junction and decreases sensitivity of postjunctional membranes to acetylcholine. In addition, magnesium increases endothelial production of prostaglandin I2 (PGI2), increases cyclic guanosine monophosphate (GMP) production, and decreases angiotensin-converting enzyme levels, actions that all promote smooth muscle relaxation and vasodilation,8 with concomitant increased uterine blood flow.
DOSING
Magnesium sulfate is usually administered as a 4-g IV bolus over 30 minutes, followed by an infusion of 1 g per hour.9 Therapeutic levels range between 4 and 9 mEq/L. Plasma levels and deep tendon reflexes must be followed rigorously to avoid overdose and complications associated with magnesium toxicity. The effects of increasing magnesium levels are listed in Table 5-1, and the treatment of magnesium toxicity is reviewed in Table 5-2. Because magnesium is excreted by the kidneys, it must be carefully titrated and monitored in patients with renal impairment.
Table 5-1. Effects of Increasing Magnesium Levels
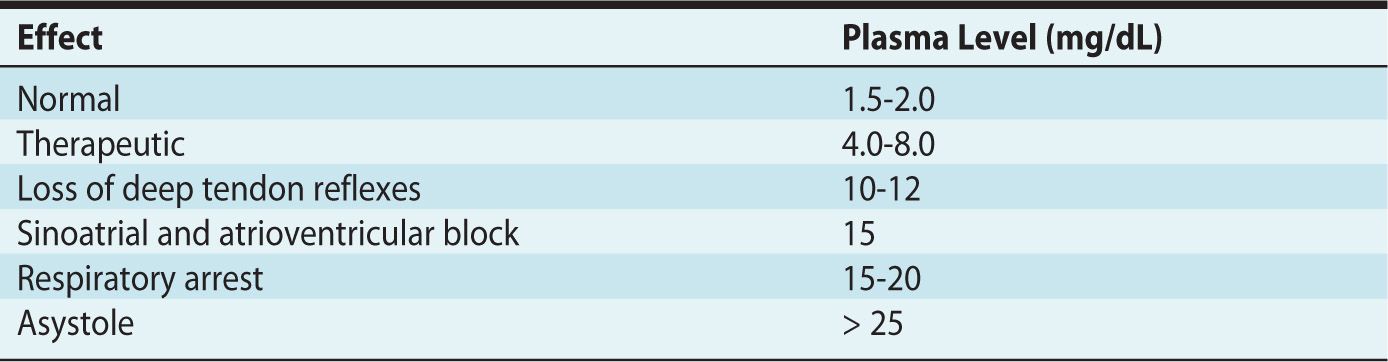
Table 5-2. Treatment of Magnesium Toxicity
ADVERSE EFFECTS
Maternal side effects range from mild to severe and occur in up to 60% of exposed women. They include flushing, nausea, blurry vision, headache, and lethargy. Besides the side effects and complications associated with magnesium toxicity, there are other important considerations when caring for patients who are receiving magnesium sulfate therapy. These are (1) decreased systemic vascular resistance and pronounced hypotension with neuraxial and general anesthesia, (2) increased risk of postpartum hemorrhage during vaginal or cesarean delivery, (3) reduction in minimum alveolar concentration of volatile anesthetics, (4) increased sensitivity of nondepolarizing neuromuscular blockers, (5) generalized muscle weakness, and (6) increased risk of pulmonary edema. All of these effects may occur at therapeutic levels of magnesium sulfate treatment and should be anticipated when magnesium sulfate is used in the peripartum course.10–12
Beta-Mimetic Medications (Ritodrine and Terbutaline)
INDICATIONS
β-Agonists are used to treat preterm labor and uterine tetany. However, β-agonists only prolong pregnancy by 24 to 48 hours, and depending on the gestational age, neonatal morbidity is not decreased.3 In addition, side effects can seriously limit the use of this class of drugs for treatment of preterm labor. For this reason, calcium channel blockers (CCBs) have replaced the use of β-mimetics to treat preterm labor in many cases. In modern-day practice, β-agonists are now more often used to treat uterine hypertonus and nonreassuring fetal status, especially following the initiation of neuraxial anesthesia. When used in these circumstances, plasma concentrations of epinephrine are increased during labor and produce tocolysis through β-agonist activity. On initiation of a combined spinal-epidural anesthetic, the rapid onset of analgesia causes an equally rapid decrease in catecholamine levels, resulting in unopposed oxytocin-induced uterine tetany and decreased placental perfusion. The β-mimetic properties of terbutaline (0.25 mg intravenously) can treat uterine hypertonus quickly and safely, but because it reaches peak effect in 1 to 2 hours can have the undesired consequence of adversely affecting a woman’s contraction pattern and labor curve.
MECHANISM OF ACTION
Uterine smooth muscle possesses β2-receptors. These are activated selectively by both ritodrine and terbutaline.9
DOSING
Although long-term oral β2-agonist therapy is ineffective in the prevention of preterm labor and delivery before 37 weeks’ gestation, the primary indications for these medications are treatment of uterine hypertonus and acute preterm labor. Doses of terbutaline 0.25 mg intravenously or subcutaneously may be repeated and titrated to a maternal heart rate 20% or 30% above baseline, but terbutaline therapy should be used with caution in patients with cardiopulmonary disease.13 In 2011, the US Food and Drug Administration (FDA) placed a Black Box Warning on terbulatine’s label stating that the medication should not be used for prolonged tocolysis (48-72 hours) because of the risk of serious maternal cardiac toxicity and death.13 Although ritodrine is the only medication approved by the FDA for tocolysis, it is no longer available in the United States.9
ADVERSE EFFECTS
The most common side effects of these drugs are related to maternal and fetal β1 stimulation of the heart and β2 effects on the pulmonary and endocrine systems. β-Mimetic agents induce transient hyperglycemia and hypokalemia. Although severe hyperglycemia is rare and typically resolves after discontinuation of β-agonist therapy, the increased blood glucose can result in hypokalemia and should be monitored closely in these patients. In some patients, the side effects of these agents may be serious and include tachycardia, myocardial ischemia, dysrhythmias, and pulmonary edema. Twin gestation, infection, and magnesium administration are risk factors for the development of pulmonary edema. β2 Stimulation can also cause hypotension and cerebral ischemia.14
Calcium Channel Blockers
INDICATIONS
Data show that CCBs are superior to β-agonists in reducing preterm labor and improving neonatal outcomes.15 They are considered first-line tocolytic agents. Nifedipine is the most commonly used CCB due to its affinity for smooth muscle over cardiac muscle.
MECHANISM OF ACTION
CCBs block calcium entry into the cell and release of calcium from the sarcoplasmic reticulum. This results in inhibition of the actin-myosin complex and produces relaxation of uterine smooth muscle.9
DOSING
Nifedipine is administered orally or sublingually, 10 to 20 mg every 4 to 6 hours.15
ADVERSE EFFECTS
Side effects are usually minimal but can include flushing, headache, and hypotension.9 The combination of CCBs and general anesthesia can produce hypotension and cardiac conduction abnormalities.16 However, severe hypotension resulting in reduced uteroplacental perfusion is rare, and adverse fetal effects have not been reported. Pulmonary edema can also occur and is more likely with concurrent administration of β-agonists and/or magnesium.17 Also, because both oxytocin and PG agonists work via calcium channels, they may be of limited use when treating hemorrhage due to uterine atony9 in patients recently treated with calcium channel blockers. In such cases, large-bore intravenous access, uterotonics, and blood products should be readily available.
Prostaglandin Synthetase Inhibitors (Nonsteroidal Anti-inflammatory Drugs)
INDICATIONS
These medications are most effective at prolonging pregnancy by 2 to 7 days in the setting of preterm labor.4 Indomethacin, sulindac, and ketorolac can be used, but their use is limited to 72 hours before 32 weeks’ gestation due to severe fetal side effects (eg, premature closing of the ductus arteriosus [after 32 weeks], oligohydramnios [with nimesulide], and necrotizing enterocolitis).18 Selective cyclooxygenase-2 (COX-2) inhibitors (rofecoxib and nimesulide) may cause fewer adverse effects than their nonselective counterparts. However, these drugs are no longer considered first-line therapy in the treatment of preterm labor.4
MECHANISM OF ACTION
PG synthetase inhibitors irreversibly inhibit COX-1 and COX-2. This prevents the production of PGE2 and PGF2α from arachidonic acid; both are smooth muscle stimulants.9
DOSING
Indomethacin is the most commonly used drug in this class and is administered orally. A 50-mg loading dose is followed by 25 mg every 4 to 6 hours for 48 to 72 hours.9
ADVERSE EFFECTS
Although maternal side effects are generally minimal, platelet dysfunction and bleeding, reduced renal perfusion, renal insufficiency, increased systemic vascular resistance, triggering of aspirin-induced asthma, nausea, and heartburn can all occur.
Nitroglycerin
INDICATIONS
Nitroglycerin (NTG) provides rapid, short-acting uterine relaxation. Although NTG can produce profound uterine relaxation, it is mainly used for procedures such as external cephalic version, manual removal of placenta, uterine inversion, head entrapment, extraction of a second twin, and reversal of tetanic uterine contraction.19 It has not been shown to be an effective treatment for preterm labor.20
MECHANISM OF ACTION
NTG increases cyclic GMP concentrations by guanylate cyclase activation and subsequent inhibition of calcium influx and smooth muscle contraction.
DOSING
NTG is administered intravenously, sublingually, or as a sublingual aerosol. When given intravenously, doses typically start at 50 μg. However, incremental doses up to 1850 μg have been reported without side effects. The dose is primarily dependent on the hemodynamic stability of the patient, with redosing dependent on hemodynamic stability.
ADVERSE EFFECTS
Transient hypotension can occur due to the effects of NTG on smooth muscle. However, due to the extremely short half-life of NTG, treatment with intravenous fluids and vasopressors are infrequently required. Maternal headache may also occur, but no adverse effects have been documented in the fetus.
Oxytocin Antagonists
INDICATIONS
Atosiban is a competitive inhibitor of oxytocin receptors and is used to treat preterm labor. Although originally thought to be superior to other tocolytics, a 2005 Cochrane database review found atosiban to be only as effective as β-agonists or placebo.21 It is considered a second-line therapy behind CCBs because of its low side-effect profile.
MECHANISM OF ACTION
Atosiban reversibly binds and inhibits decidual and myometrial oxytocin receptors.9
DOSING
Atosiban is administered as an intravenous infusion at 300 μg/min.9
ADVERSE EFFECTS
Although atosiban binds very selectively to oxytocin receptors and the myometrium remains sensitive to oxytocin after atosiban administration, it has minimal maternal side effects. It does not cross the placenta and has no effect on the fetus.
Uterotonic Medications
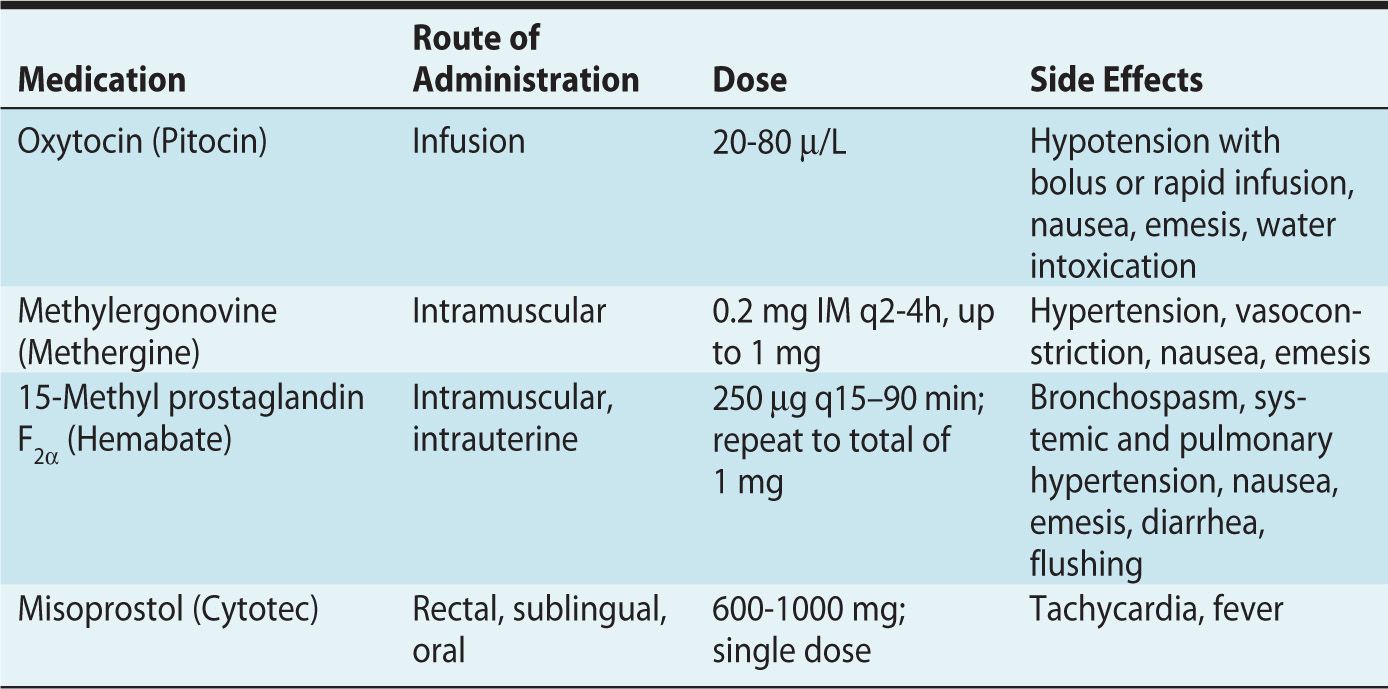
Uterotonics increase contraction and tone of the uterus and are most commonly used to treat uterine atony (Table 5-3). Uterine atony is the most common cause of postpartum hemorrhage22 and a leading cause of maternal mortality in the postpartum period. Judicious and timely use of these medications is often necessary.23 Other uses include cervical ripening, induction of labor, or termination of pregnancy. Three classes of uterotonics are currently used: oxytocin, ergot alkaloids, and PGs.
Table 5-3. Uterotonics
Oxytocin
INDICATIONS
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








