TOPICS
Neurologic and neuromuscular disorders can significantly complicate the clinical management of the obstetric patient. These disorders may have an impact on the course of pregnancy and/or lead to substantial challenges in the delivery of the parturient. A thorough assessment of the patient in collaboration with the neurology and obstetric services is necessary to provide optimum care. This chapter outlines the anesthetic considerations in women with common neurologic and neuromuscular disorders. Although the discussion is by no means exhaustive, the selected disorders may have significant impact on the anesthetic and obstetric management of parturients.
NEUROLOGIC DISORDERS
Multiple Sclerosis
EPIDEMIOLOGY
Multiple sclerosis (MS) belongs to a group of disorders characterized by abnormalities in the myelin sheath from either defective synthesis or loss of myelin postneuronal development. MS is the most common demyelinating disorder, with a prevalence of greater than 1 million individuals affected worldwide. Of these, 400,000 reside in the United States. Women are more commonly affected than men, with a mean onset age of 30 years. As a result, MS may occur during reproductive age. MS does not affect the peripheral nervous system and usually does not have a negative impact on fertility or pregnancy outcome. Indeed, most MS patients can maintain a high level of function for many years after initial diagnosis.
ETIOLOGY/RISK FACTORS
The clinical course of MS is highly variable and is characterized by either exacerbating-remitting or chronic progressive patterns of disability. Clinical symptoms depend on the location of the demyelinating lesions and may include muscle weakness, visual disturbances, paresthesia, loss of balance, fatigue, bowel or bladder dysfunction, cognitive impairment, and cerebellar manifestations (such as ataxia, slurred speech, and intention tremors). Severe respiratory complications can also occur from respiratory and bulbar muscle weakness.1
Diagnosis is based on the clinical presentation as well as diagnostic testing, such as magnetic resonance imaging (MRI) (Figure 25-1), computed tomography (CT), visual evoked potentials, and lumbar puncture for cerebrospinal fluid (CSF) testing for elevated immunoglobulin levels and specific oligoclonal bands of immunoglobulin G (IgG). The detection of plaques on MRI due to focal loss of myelin in white matter can confirm the diagnosis but may not always correlate with the severity of the disease (Figure 25-1).2
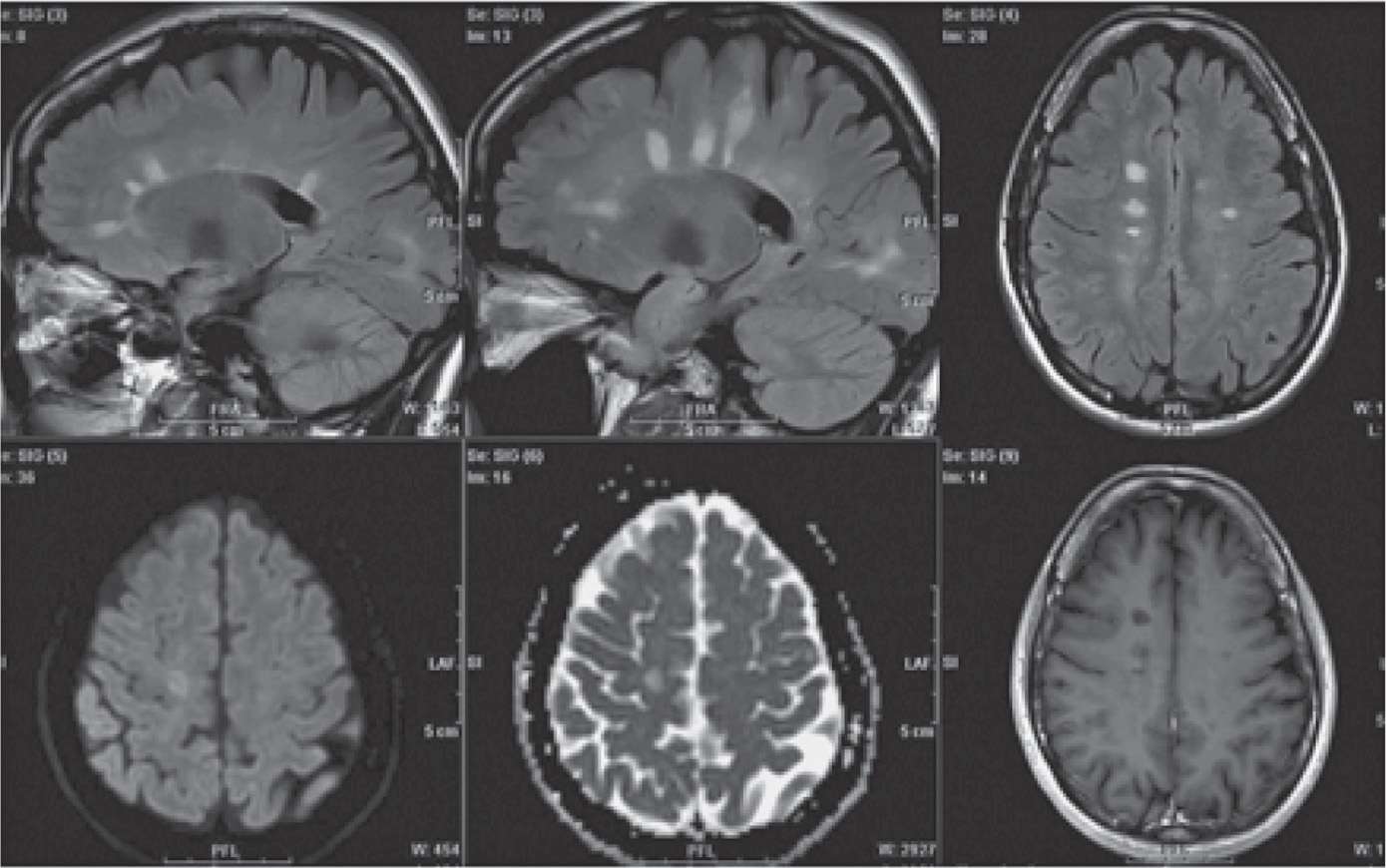
Figure 25-1. Magnetic resonance imaging scans include white matter plaques in multiple sclerosis. (Permission granted for Lövblad KO, Anzalone N, Dörfler A, et al. MR imaging in multiple sclerosis: review and recommendations for current practice. AJNR Am J Neuroradiol. 2010;31(6):983–989 © by American Society of Neuroradiology.)
Although the cause of MS is still not known, several genetic factors, environmental factors, viral or infectious exposure, and autoimmune etiologies have been proposed. The disease is thought to develop from a complex interplay between genetic and environmental risk factors. Ethnicity and genetic risk factors, such as certain human leukocyte antigen (HLA) patterning, have been associated with the risk for developing the disease. The strongest environmental risk factors for MS identified so far include Epstein-Barr virus infection, smoking, and vitamin D deficiency. Certain conditions such as infection, fever, surgery, and emotional stress may adversely influence the clinical course of the disease.3
PATHOPHYSIOLOGY
MS is a chronic, progressive, degenerative disease of the central nervous system characterized by focal or segmental demyelination, resulting in axonal damage and loss, with gliosis in the brain and spinal cord. Demyelination leads to conduction blockade that fluctuates, resulting in varying clinical symptoms with relapses and remissions.
MANAGEMENT
There is at present no cure for MS. Treatment strategies focus on symptom control and slowing disease progression by using immunosuppressive and anti-inflammatory drugs. The management options include corticosteroids, interferon β-Ia, interferon β-Ib, glatiramer acetate, methotrexate, and mitoxantrone, several of which are contraindicated for pregnancy, especially during the first trimester (all of which are at least pregnancy category C).4
Many MS patients are able to reduce or stop their usual MS therapy during pregnancy because pregnancy itself may lead to immunosuppression. There is no significant increase in spontaneous abortion rate, or other major obstetric or neonatal complications for MS parturients, as compared to women without MS. However, there is an approximately 10% increase in premature delivery and a less significant increase in instrumental delivery and cesarean section in women with MS as compared to parturients without the disease.5 Glucocorticoids are recommended for treatment of acute relapses during pregnancy and parturition.
ANESTHETIC CONSIDERATIONS
Parturients with MS should be evaluated for the chronology and pattern of MS symptoms, medications, and adequacy of respiratory function. Regardless of the anesthetic technique selected, frequent monitoring of body temperature is essential, because a single-degree rise in temperature may cause relapse or worsen existing symptoms. Thus, even mild temperature elevation should be aggressively treated with antifebrile agents. Regional anesthesia has not been reported to adversely affect the disease and may even be beneficial by mitigating the stress response during labor and delivery. However, controversy exists as to whether exposure to local anesthetics negatively impact demyelinated neurons. Based on this, the recommendation is that the lowest effective concentration of local anesthetic be used, combined with opioids, and that patient temperature be monitored, with any elevations treated aggressively. This is important because there has been conflicting evidence that epidural analgesia during labor may result in small temperature increases. In the past, small retrospective and observational studies suggested that any type of neuraxial anesthesia for labor and delivery would result in a 30% relapse rate of MS in the puerperium. However, in 2004, a large prospective study found that the relapse rate among woman with MS was 30% 3 months postpartum; regardless of anesthetic technique, or even if no anesthetic was administered.6
Cesarean delivery should be reserved for obstetric indications only. Epidural or combined spinal-epidural anesthesia may be safely used for cesarean section in parturients with MS. However, additional precautions need to be taken when general anesthesia is used. Succinylcholine should be avoided, with its potential to cause hyperkalemia, particularly if there are muscle-wasting symptoms present. Parturients with MS, particularly those on baclofen, may exhibit abnormal responses to nondepolarizing muscle relaxants. Accordingly, dosing of nondepolarizing muscle relaxants should be guided by neuromuscular monitoring.
POSTPARTUM CARE
MS patients require careful monitoring postdelivery, including monitoring of body temperature and hemodynamic variables. In addition, careful attention to pain relief will reduce stress and play a part in preventing exacerbations. There may be a risk of airway compromise, hypoventilation, and atelectasis if the bulbar or respiratory musculature is affected by MS. Residual neuromuscular blockade must be treated aggressively. A complete neurologic evaluation should be performed at intervals in the immediate and 3 months’ postpartum period to assess any exacerbation of the MS. The patient should be restarted, as soon as possible, on her prepregnancy MS drug regimen. Exacerbations should be treated as described earlier, including intravenous immunoglobulin and steroids. There are no data to prevent or recommend breastfeeding with respect to the course of MS.5 In some cases, it may not be recommended to breast feed when drugs that may affect the neonate are secreted in the breast milk.
Guillain-Barré Syndrome
EPIDEMIOLOGY
Guillain-Barré syndrome (GBS) can be an acute or subacute inflammatory demyelinating peripheral polyneuropathy, which is typically triggered by an acute infection. It initially presents with distal paresthesias and weakness, progressing to ascending paralysis. GBS incidence is reported to be 1.7 cases per 100,000 of the population7 and fortunately is relatively rare during pregnancy.
ETIOLOGY/RISK FACTORS/PATHOPHYSIOLOGY
GBS is diagnosed by clinical presentation, CSF analysis (elevated CSF protein concentration occurs in 80% of cases), and electrodiagnostic tests such as nerve conduction studies. Many patients with GBS have an antecedent viral infection, such as cytomegalovirus, Epstein-Barr virus, and varicella zoster (most common), or a bacterial infection, such as with Campylobacter jejuni, Mycoplasma pneumoniae, or Haemophilus influenzae. The proposed mechanism is that infectious agents induce autoantibody production that cross-react with peripheral nerve myelin components, such as specific gangliosides and glycolipids (Figure 25-2).8 In its most severe form, the disease may result in life-threatening complications by affecting the respiratory muscles or the autonomic nervous system.
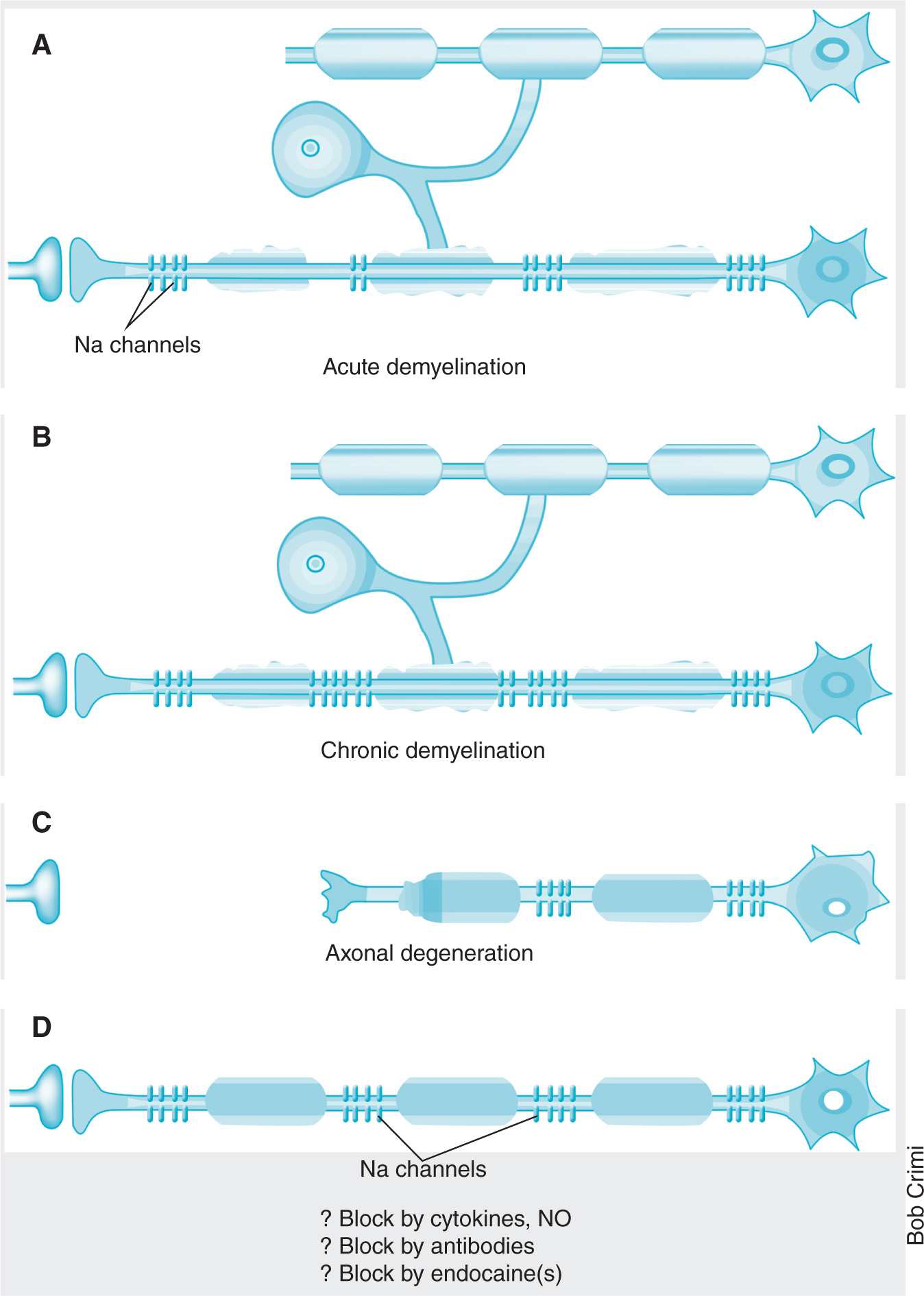
Figure 25-2. Mechanisms of dyemyelination. (Reprinted by permission from Macmillan Publishers Ltd: Waxman SG. Do “demyelinating” diseases involve more than myelin? Nat Med. 2000;6(7):738–739. Copyright 2000.)
MANAGEMENT
There is no cure for GBS, and treatment is supportive. During pregnancy, patients with GBS may require ventilator support to improve maternal outcome and maximize uteroplacental oxygen delivery. GBS, in of itself, is not an indication for cesarean section, and normal spontaneous vaginal delivery has been reported. However, the ability to bear down (stage 2) may be weakened, so vacuum-assisted extraction at delivery may prove beneficial or necessary. High-dose intravenous pooled IgG plasmapheresis may be useful and is not contraindicated during pregnancy.
ANESTHETIC CONSIDERATIONS
Parturients with GBS usually are managed with supportive therapy. It is important to consider that an incremental acute strain, related to labor and delivery, in an otherwise stable patient may intensify the need for supportive therapy, including ventilation. Thromboembolic prophylaxis is important particularly in bedridden patients. The decision to select general versus regional anesthesia should be carefully considered in all patients with respiratory compromise. Both spinal and epidural anesthesia have been used successfully in parturients with GBS for vaginal delivery as well as for cesarean section. There is one reported case of a woman with GBS having worsening neurologic symptoms that was temporally related to an epidural anesthetic.9
Furthermore, autonomic instability may occur as a result of GBS, and there is the potential for local anesthetic–induced sympathectomy, which can result in profound hypotension and bradycardia, rarely leading to cardiovascular collapse. In addition, patients with GBS may have reduced pain perception due to denervation and thus require lower doses of local anesthetic for a comparable block to women without GBS. Succinylcholine should be avoided during general anesthesia, regardless of whether GBS has resolved, as it may lead to hyperkalemia and potential progression to cardiac arrest.7
POSTPARTUM CARE
Obstetric outcome is generally good, although there may be an increased risk of preterm delivery and the rare occurrence of neonatal GBS due to placental transfer of maternal autoantibodies.9 Postpartum care of the parturient is similar to that for other demyelinating lesions and should be supportive.
Chiari Malformation
EPIDEMIOLOGY
Chiari malformation (CM) is a congenital anomaly where there is a downward displacement of the cerebellar tonsils through the foramen magnum (Figure 25-3). The incidence of CMs (types I and II) is estimated to occur in 1% of all live births. The actual incidence could be higher, because patients with type I CM can be asymptomatic or have few subtle symptoms for many years prior to diagnosis.
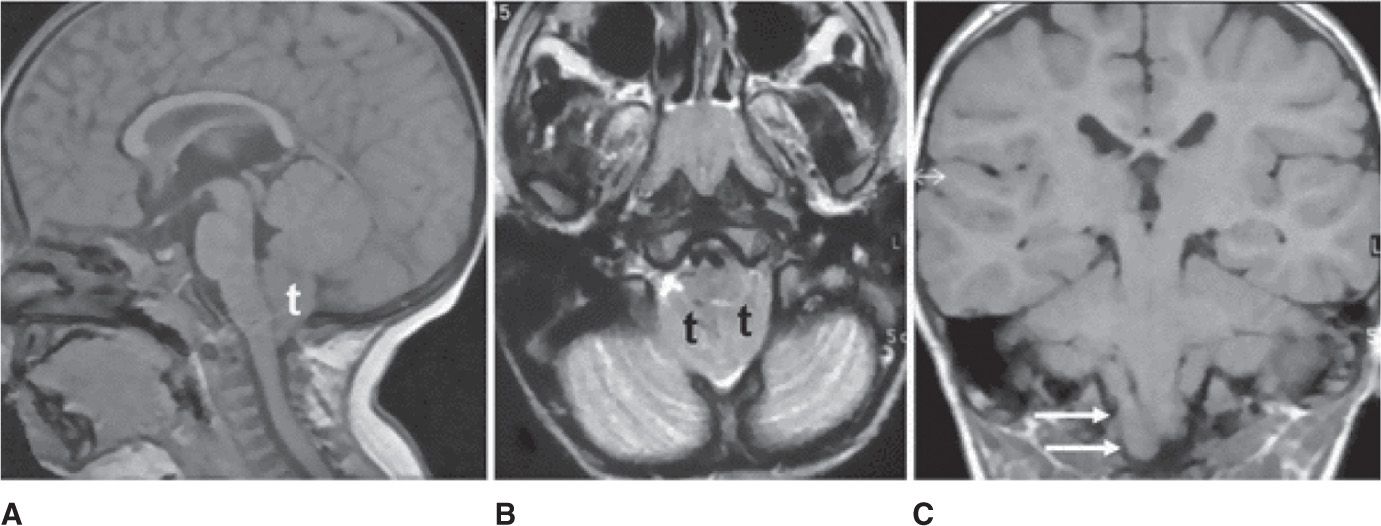
Figure 25-3. Magnetic resonance images showing Chiari malformation. Sagittal T1-weighted image A demonstrates herniation of the cerebellar tonsils (t) through the foramen magnum into the cervical spinal canal. Axial T2-weighted image B shows crowding of the foramen magnum due to the presence of the tonsils (t) associated with thinning of subarachnoid spaces. In coronal T1-weighted image C, tonsillar ectopia is asymmetric with low position of the right (arrows). (Permission granted for Chiapparini L, Saletti V, Solero CL, Bruzzone MG, Valentini LG. Neuroradiological diagnosis of Chiari malformations. Neurol Sci. 2011;32(suppl 3):S283-S286. With kind permission from Springer Science and Business Media.)
ETIOLOGY/RISK FACTORS/PATHOPHYSIOLOGY
The underlying cause of CM is unknown, with possible risk factors identified as vitamin/nutrient deficiencies, hazardous chemical exposure, infection, drug use, or alcohol consumption during pregnancy. The condition is classified into four types based on progressive degrees of severity.10 Type I, the least severe form, presents with symptoms in young adults and 25% of cases have syringomyelia. Type II (also known as Arnold-Chiari malformation) presents in infancy. The defect includes a downward displacement of the cerebellar vermis, brainstem, and fourth ventricle, with formation of hydrocephalus and myelomeningocele. Multiple surgeries may be required and these children will likely have developmental delay as well as physical disabilities. Tragically, there is a 50% mortality in early childhood. Type III and Type IV are rare malformations with very poor prognoses, due to herniation and cervical myelomeningocele with fourth ventricle hydrocephalus or cerebellar hypoplasia without herniation, respectively.
The common symptoms associated with CM are severe occipital headache, numbness, tingling in the neck and arms, pain, syncope, dizziness, vertigo, tinnitus, nystagmus, and fatigue. Syringomyelia is an abnormal expanded cystic cavity within the spinal cord filled with CSF, which leads to disruption of neural tissue function. Symptoms of syringomyelia include weakness, burning pain in the neck or back, extremity paresthesia, and referred chest pain. There is also involvement of paraspinal muscles causing weakness, kyphoscoliosis, restrictive pulmonary function, and vocal cord abductor paresis. Autonomic dysfunction manifests as delayed gastric emptying, urinary bladder dysfunction, and impaired thermoregulation. MRI is the preferred diagnostic test, in concert with patient history and neurologic examination. Surgery may be necessary for posterior fossa decompression and duraplasty, particularly in patients with progressively worsening symptoms, hydrocephalus, or syringomyelia. In cases where progressive hydrocephalus occurs, shunts may be required.11
MANAGEMENT
Pregnancy itself does not have an influence on the course of the disease; however, parturients with CM and/or syringomyelia should be counseled carefully regarding the possible risk of increased intracranial pressure (ICP) during pregnancy and labor.
ANESTHETIC CONSIDERATIONS
Parturients with CM type I should be evaluated for signs of increased intracranial pressure (ICP) and neurologic deficits. Factors that may increase ICP, such as coughing, hypercapnia, and pushing with prolonged straining during the second stage of labor, should be minimized because they may cause increased risk of herniation or worsening of symptoms.12 For those parturients with normal ICP and no other neurologic symptoms at the time of delivery, epidural analgesia has been safely administered. It is prudent to avoid transmitted increases in ICP by slowly administering local anesthetic to avoid sudden distention of the epidural space and avoiding sudden drops in arterial pressure if the patient has autonomic neuropathy. Epidural, spinal, and general anesthesia have all been used successfully for cesarean delivery in patients with CM who have normal ICP.13
Spinal anesthesia should be avoided in parturients who have CM with coexisting syringomyelia, regardless of ICP. For those women with symptoms of increased ICP, the mode of delivery should be either by planned cesarean delivery or a controlled labor with an abbreviated second stage culminating in assisted vaginal delivery. In patients with elevated ICP, dural puncture, either from spinal or accidental dural puncture from an epidural needle, may lead to herniation of the brain due to sudden excessive loss of CSF. Other forms of analgesia, such as lumbar sympathetic blocks, or intravenous patient controlled analgesia, should be considered, even if they are less effective than neuraxial techniques.13 For cesarean delivery, general anesthesia should be considered in those women with elevated ICP, but precautions should be taken, such as hyperventilation, adequate depth of anesthesia during laryngoscopy/intubation, arterial line placement to monitor swings in blood pressure, and careful attention to head positioning to avoid neck hyperextension. In the presence of neurologic symptoms, there may be an increased sensitivity to succinylcholine administration with a potential altered response, and conversely, resistance to nondepolarizing muscle relaxants. Therefore, neuromuscular blockade should be monitored at all times and the need for postoperative ventilation considered.14
POSTPARTUM CARE
The goal of postpartum care is to prevent increases in ICP. Monitoring of neurologic status is essential in the immediate postpartum period, effective pain relief is required to prevent wide fluctuations in blood pressure that can impact ICP.15
Seizure Disorders
EPIDEMIOLOGY
More than 1 million women of reproductive age in the United States suffer from a seizure disorder; approximately 20,000 of whom give birth each year. Preexisting epilepsy is the most common cause of seizures during pregnancy, with eclamptic seizures the second. Less common causes include traumatic brain injury, brain tumor, stroke, electrolyte/metabolic abnormalities, and drug withdrawal. Epilepsy is a disease in which the patient develops recurrent seizures in the absence of an identifiable cause. The incidence of epilepsy in the general population is approximately 1% to 2% and has been reported to affect up to 0.7% of pregnancies.16
ETIOLOGY/RISK FACTORS
Seizures are grouped into two categories: generalized seizures (grand mal) and partial seizures (petit mal). Generalized seizures are produced by abnormal electrical impulses involving and spreading to the entire brain, whereas partial seizures involve just a focal area. Grand mal seizures are characterized by loss of consciousness, followed by “tonic” and “clonic” convulsions and then a “post-ictal” phase. The underlying cause of epilepsy is unknown, but it is likely due to a combination of environmental and genetic factors. Genetics may also influence the efficacy of antiepileptic drug.
PATHOPHYSIOLOGY
Seizures are characterized by abnormal, hyperexcitable neuronal firing, leading to hypersynchrony within the brain, which can affect motor control, sensory perception, behavior, and/or autonomic function. Defects in neuronal function, such as mitochondrial dysfunction, glia-mediated excitation, inflammation, and blood-brain barrier impairment have been implicated in the pathophysiology of epilepsy.17
MANAGEMENT
Although there are several drugs available to treat seizures, many patients suffer from refractory symptoms and/or have reduced quality of life due to side effects of anticonvulsant medications. Pregnancy can have a variable effect on seizure frequency; in some woman there may be up to a 30% increased frequency of seizures while pregnant. However, the likelihood of seizures may be lower during pregnancy if the woman was without seizures in the year leading up to pregnancy.18 Serum levels of anticonvulsants may be reduced during pregnancy due to vomiting, delayed intestinal absorption, altered protein binding, folic acid supplementation, alterations in pharmacokinetics, and increased renal clearance. Higher estrogen concentration during pregnancy and the potential for alkalosis secondary to hyperventilation can lower the seizure threshold. There may be decreased maternal compliance in adherence to anticonvulsant regimens out of concerns for teratogenicity or other adverse fetal effects. Due to physiologic changes associated with pregnancy that may affect drug disposition, pregnant women require close monitoring of therapeutic anticonvulsant levels.
Some anticonvulsants have the potential for teratogenicity, particularly those with antifolate properties. When pregnancy is anticipated, it is prudent to convert to accepted anticonvulsant monotherapy that is not associated with teratogenicity. Administration of folate prior to conception and during pregnancy has been recommended to reduce the risk of fetal neural tube defects in woman taking anticonvulsants.19
There is controversy as to whether anticonvulsant drugs increase the risk of spontaneous hemorrhage in newborns due to increased vitamin K metabolism. Accordingly, oral vitamin K 10 to 20 mg recommended for pregnant women taking anticonvulsant drugs and 0.5 to 1 mg of vitamin K intramuscularly for newborns, immediately after delivery.20
Seizures during pregnancy may result in maternal and fetal risk. Generalized tonic-clonic seizures are especially dangerous to the developing embryo and fetus, because the potential for maternal hypoxia and hypercapnia during periods of apnea, can result in intrauterine fetal asphyxia. A generalized tonic-clonic seizure can also cause trauma, spontaneous abortion or placental abruption, fetal intracranial bleeding, fetal heart rate changes, and even intrauterine fetal death. Seizures occurring in a pregnant woman should be treated rapidly, with special care given the potential for aspiration, oxygenation and ventilation to prevent maternal hypoxia, left uterine displacement, and termination of the seizure with a small dose of rapidly acting anticonvulsant.
ANESTHETIC MANAGEMENT
Parturients with epilepsy have a higher risk of pregnancy-related hypertension, preeclampsia, preterm labor, bleeding in pregnancy, and an increased risk of cesarean delivery compared to nonepileptic parturients. All epileptic parturients should have an anesthesiology consultation early in the course of their pregnancy. Anticonvulsant medication levels should be assessed and if the serum drug level is inadequate, patients should be given supplemental doses of anticonvulsant or administered parenteral agents (eg, phenytoin) (to avoid unpredictable gastric absorption during labor).
Some anticonvulsant drugs have potential interactions with anesthetic medications. Phenytoin and phenobarbital induce hepatic microsomal enzymes, which increase the metabolism and breakdown of opioids, nondepolarizing muscle relaxants, and volatile anesthetic agents. Meperidine and ketamine can lower the seizure threshold and should be avoided if feasible. Neuraxial analgesia or anesthesia may be administered safely in epileptic parturients.21 Epidural analgesia is indicated for vaginal delivery to decrease the requirement for systemically administered drugs and narcotics, relieve pain, reduce hyperventilation from uterine contractions, and reduce physical and emotional stress and fatigue; all of these may decrease the seizure threshold.
Cesarean delivery should be performed exclusively for obstetric indications. For elective cesarean delivery, both spinal and epidural anesthesia can be used successfully. General anesthesia may be necessary if the patient is in status epilepticus (intractable seizures either as a continuous seizure or a lack of full recovery between seizures (postictal stage). In these situations, it is important to protect the airway; ensure oxygenation and ventilation; and treat seizures pharmacologically with benzodiazepines, phenytoin, or phenobarbital. The fetus must be monitored and if there is a nonreassuring fetal status, there should be attempts at intrauterine resuscitation. In most cases, return of maternal oxygenation and ventilation, as well as cessation of the seizures, will usually result in improving fetal status. Rarely, an emergent cesarean delivery must be attempted. Some anesthetic agents, such as ketamine, meperidine, etomidate, methohexital, and phenothiazines should be avoided, because they are epileptogenic, particularly in the presence of hypocapnia. Certain volatile agents (sevoflurane more than isoflurane) are epileptogenic, whereas others, such as nitrous oxide, may suppress seizure-like activity. Thus, a combination of isoflurane and nitrous oxide may be a preferred combination when general anesthesia is required.22 Because phenytoin and phenobarbital increase the metabolism and breakdown of nondepolarizing muscle relaxants, their doses needs to be adjusted accordingly.
POSTPARTUM CARE
Postdelivery, anticonvulsant medications should be continued with monitoring of therapeutic levels as indicated. There may also be an increased risk of postpartum bleeding.16 Epidural morphine for postpartum pain relief should be used with caution because there are reports of seizure activity following its use.23 Parturients with epilepsy may breastfed, and newborns should be monitored for any acute changes in behavior, such as lethargy/poor feeding. If these occur, serum anticonvulsant levels should be checked. There may be an increased risk of respiratory distress in neonates from mothers taking antiepileptic drugs.24
Spinal Cord Injury
EPIDEMIOLOGY
In the United States, approximately 2000 new spinal cord injuries (SCIs) occur in young women each year,21 some of whom may be pregnant. In addition, improved medical care and survival have resulted in a higher number of women with SCIs who present for obstetric care.
ETIOLOGY/RISK FACTORS
The most common cause of SCIs include motor vehicle collisions (40%), diving accidents, gunshot wounds, MS, spinal cord hematomas, and transverse myelitis.25 Spinal cord injuries above the T1 level result in paralysis of all four extremities (quadriplegia), whereas injuries below T2 usually cause paralysis of only the lower extremities (paraplegia).
Immediately after SCI, spinal shock can occur as a result of sympathetic denervation below the level of the injury, which then leads to generalized peripheral vasodilation, severe hypotension, and flaccid paralysis, with loss of tendon and autonomic reflexes. For lower level SCIs, reflex tachycardia can occur, in response to hypovolemia and hemodynamic instability in quadriplegic patients, with significant circulatory collapse developing in the absence of brainstem regulation of vasomotor tone. Alternatively there may be paradoxical bradycardia and there is often altered temperature regulation, sweating, and piloerection, which can last for 1 to 3 weeks after the injury due to loss of vasomotor tone. During spinal shock any slight change in position, Valsalva maneuver, or tracheal suctioning, can produce profound hypotension and bradycardia as a result of unopposed vagal stimulation.
Over time, SCI patients can maintain blood pressure through enhanced activation of the renin–angiotensin–aldosterone system, which may later play a role in the development of autonomic dysreflexia (ADR), a potentially life-threatening complication of SCI.25
PATHOPHYSIOLOGY
Brain and spinal cord tissue are very susceptible to traumatic injury with little or no ability to regenerate and repair. There are two major phases to SCI. Initially, in the acute phase, the injury leads to necrotic cell death, edema, and ischemia. Subsequently, a second injury cascade consisting of inflammation, release of free radicals and cytotoxic levels of excitatory amino acids (Figure 25-4).26 The loss of white matter is a primary cause of neurologic dysfunction and is a major focus of research for therapeutic targets, many of which are geared to preventing the secondary excitatory damage phase and then promoting regenerative function that remains in the neuronal tissue.
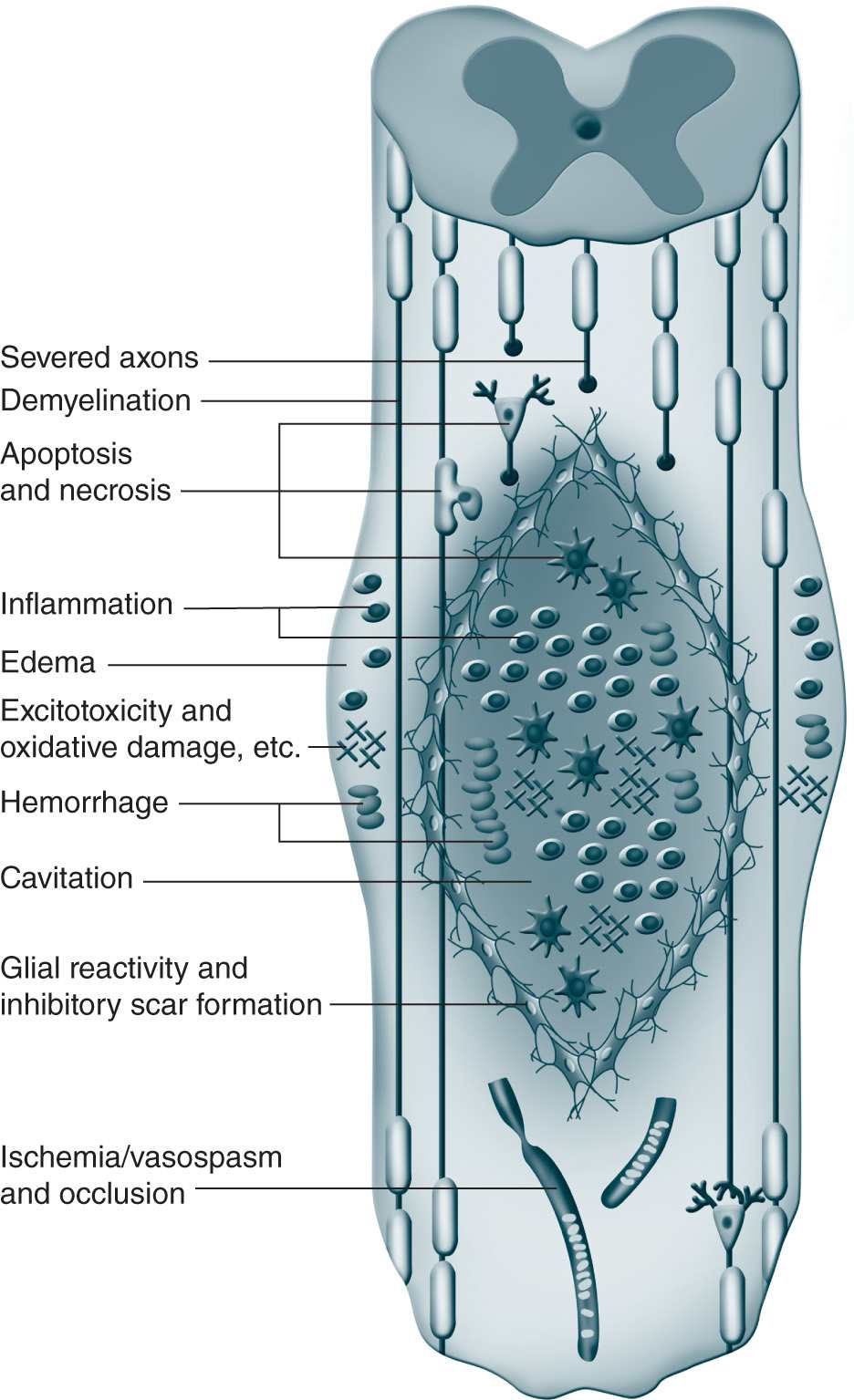
Figure 25-4. Pathophysiology of spinal cord injury. (From Mothe AJ, Tator CH. Advances in stem cell therapy for spinal cord injury. J Clin Invest. 2012; 122(11):3824-3834. Reproduced with permission of the American Society for Clinical Investigation.)
MANAGEMENT
There is no current cure for SCI, and most SCI patients have significant disability. However, much research is focused on restoring function using multifactorial strategies, such as stem cell therapies.26 Current treatment options include surgery to decompress and stabilize the injury, prevention and management of secondary complications, and rehabilitation.
Autonomic dysreflexia (ADR) or hyperreflexia is a serious, life-threatening complication of SCI. The onset of ADR following SCI is variable, affecting up to 85% of patients with an injury above the T6 level. ADR can be triggered by any stimulus, including bladder or bowel distention, pressure sores, or labor pain, any of which can lead to extreme sympathetic hyperactivity and severe systemic hypertension. In patients with SCI at T6 or below, there can be a compensatory reflex parasympathetic response, leading to bradycardia and vasodilation above the level of the injury, but injuries above T6 have insufficient autoregulatory mechanisms to compensate for the severe hypertension that may develop. The most common sequelae for ADR include severe systemic hypertension, hyperthermia, piloerection, increased extremity spasticity, respiratory distress, loss of consciousness, convulsions, intracranial hemorrhage, dysrhythmias, myocardial infarction, and in some cases mortality.27 Immediate diagnosis and treatment can be life saving. Management includes ceasing the noxious stimulus, if feasible, and the use of rapid onset and shorter acting antihypertensive agents such as sodium nitroprusside, sublingual nitroglycerin, nifedipine, and/or hydralazine to control cardiovascular manifestations. Labetalol may be a more preferable choice for parturients. All these antihypertensive medications must be used judiciously because of the potential to cause acute hypotension that could diminish fetal blood flow.
ANESTHETIC CONSIDERATIONS
Acute SCI above C4 may require immediate intubation for respiratory support, because innervation to the diaphragm is likely to be compromised. A hard cervical collar should be used to limit neck movement. Intubation may be accomplished by awake fiber optic intubation or by direct laryngoscopy with “in line stabilization” of the head and neck. In parturients at 24 weeks’ gestation or beyond, a small wedge placed to achieve 10 degrees or more of lateral uterine tilt to prevent aortocaval compression by the gravid uterus is recommended.
Immediate volume resuscitation and pressor support is needed in the acute phase of spinal shock. Signs of internal hemorrhage can be masked by spinal shock symptoms, and proper evaluation of the abdomen to exclude hemorrhage may also be difficult due to the gravid uterus. Serial hematocrit measurements, diagnostic peritoneal lavage, and a high index of suspicion for internal hemorrhage are necessary. Cardiotocography can be useful in the setting of acute SCI for fetal heart rate tracing and uterine contraction monitoring. After initial stabilization, management includes measures to prevent pulmonary infections, urinary tract infections, decubitus ulcers, constipation, anemia and respiratory failure, and prophylactic anticoagulation treatment, as there is increased risk of deep vein thrombosis and pulmonary embolism. These parturients have a higher risk of spontaneous abortion, congenital fetal malformation, preterm labor, placental abruption21 and also increased risk for unattended delivery, secondary to unrecognized uterine contractions.
General anesthesia may be preferred over regional anesthesia for surgery in acute SCI parturients, because optimal positioning of the patient may limit placement of neuraxial techniques. The time frame for the upregulation of acetylcholine receptors can be variable; thus, succinylcholine should not be administered after SCI because of the potential for uncontrolled hyperkalemia. Accordingly, high-dose nondepolarizing muscle relaxants or fiberoptic awake intubation should be utilized for intubation. A deep plane of anesthesia is needed to limit ADR with vigilant monitoring for hypotension, dysrhythmia, and uterine atony.28 Regional anesthesia, if positioning is possible, has also been used. Proper hydration and monitoring are required to prevent hypotension. The sensory level must definitively reach a T6 level to prevent ADR.
Patients with a previous SCI should have prenatal anesthesia consultation to establish an anesthetic management plan for labor and delivery, including multidisciplinary management in a high-risk obstetric unit. Regional anesthetic techniques with a continuous intrathecal catheter are preferred for incremental dosing because these avoid the risk of major hypotension and limit the chance of a high spinal. To prevent hypotension, adequate prehydration is crucial before initiating regional anesthesia and any manifestation of hypertension after regional placement can be indicative of an inadequate block and activation of ADR. Epidural anesthesia using a combination of a local anesthetic with an opioid, but not opioids alone, can be very effective in blocking afferent impulses below the level of SCI and preventing ADR. Epidural anesthesia is preferred but may be difficult to place in parturients with previous spinal surgery/instrumentation, and so continuous spinal is also another option to consider. Epidural placement should be considered prior to the onset of labor for parturients with a high risk of developing ADR. Continuous hemodynamic monitoring by electrocardiogram, pulse oximetry, or arterial line may be necessary during labor, depending on hemodynamic stability. The rate of spontaneous vaginal delivery is higher in parturients with SCI below the T6 level, whereas the rate of assisted vaginal and caesarean delivery is higher in patients with spinal cord lesions at or above the T6 level. The latter are more likely to develop ADR.
Acute onset of ADR during labor can be difficult to distinguish from preeclampsia, but delay in diagnosis can result in decreased uteroplacental blood flow, fetal hypoxemia, and fetal bradycardia. Antihypertensive medications should be readily available for use. Both spinal and epidural anesthesia can provide protection against ADR during cesarean delivery. For emergent cesarean delivery, general anesthesia may be required, with the same considerations as in acute SCI parturients, although succinylcholine may not be as problematic 1 year after the SCI.28
POSTPARTUM CARE
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







