Fig. 32.1
Clinical decision tree to assess etiology of symptoms of intrathecal medication withdrawal
32.3 Intrathecal Withdrawal
There are only three drugs that are FDA approved for intrathecal delivery: morphine, baclofen, and ziconotide. There is no recognized withdrawal syndrome associated with ziconotide. Withdrawal from intrathecally administered opioids presents in a similar fashion as other routes of opioid administration; however, the time course may differ. The sudden halting of intrathecal baclofen administration can result in a severe withdrawal syndrome that may be much worse than seen with halting oral administration. Intrathecal baclofen withdrawal could potentially be life-threatening. In addition to these three FDA-approved drugs, there are a number of other agents that have been routinely used off-label in intrathecal pumps. These medications include the opioids hydromorphone, sufentanil, and fentanyl as well as adjuvant analgesics bupivacaine and clonidine. There is no withdrawal from bupivacaine. Clonidine withdrawal presents as hypertension and tachycardia and can be severe.
32.4 Drug Mixing Error
Any new symptoms, including signs of withdrawal, which occur shortly after a pump refill, should raise concern for a possible drug mixing error. This risk is potentially higher in patients whose intrathecal medication is compounded. Patients on simple regimens of morphine or baclofen at standard premixed concentrations do not require compounding but could have the wrong drug placed into their pump. Compounding of intrathecal medications is the added step of a pharmacist producing a special formulation of multiple drugs and/or non-standard concentrations for a single patient. Many physicians will mix multiple drugs together for infusion intrathecally, thus requiring a compounding pharmacy. Additionally, those patients who require nonstandard drug concentrations also must have the drugs produced by a compounding pharmacist. Errors in mixing these compounded solutions could result in either overdose or underdose and withdrawal.
When a drug mixing error is suspected as a potential cause of intrathecal withdrawal, the first step is to replace the reservoir volume of drug with a new mixture. After removing the previous solution, it may be worth sending a sample for analysis by mass spectroscopy to determine the drugs and concentrations present in the solution. This may not always be feasible though as this is often a costly test that may take several days to receive results. Replacing the drug in the pump reservoir does not remove and replace the volume of drug in the internal tubing of the pump. As a result, it will take time for the new medication to be pumped through the tubing and catheter into to the cerebrospinal fluid. However, drug diffusion and mixing between the reservoir and catheter will rapidly equilibrate the concentration resulting in a near-normal drug dose delivery.
32.5 Pocket Fill
A “ pocket fill” is the inadvertent injection of medication into the pocket or space surrounding the intrathecal pump, instead of into the pump reservoir. A pocket fill can occur if the needle is not inserted through the refill port septum until it has reached the needle stop at the back wall of the reservoir. Tactile feedback is paramount to a correctly performed refill. Without this feedback, a clinician may not realize that the needle is incorrectly positioned [1]. Properly placed needles can also inadvertently be withdrawn from the reservoir after correct placement and before drug is injected. In either case, the injected medication would either be delivered into the subcutaneous tissue around the pump pocket or within the pump pocket instead of the pump reservoir [1].
Patients with a pocket fill can show symptoms of either an overdose or underdose. An overdose usually presents quickly in a matter of minutes to hours [1]. Low-concentration intrathecal drugs are more likely to result in underdose instead of overdose. For instance, 20 mL of baclofen 500 mcg/mL injected into a pump pocket would result in systemic delivery of only 10 mg of baclofen. Similarly, 20 mL of morphine 0.5 mg/mL would result in a systemic dose of morphine of just 10 mg. An underdose can become clinically significant if a pocket fill goes unrecognized and the pump empties sooner than anticipated [1]. This interruption of therapy usually presents in several days to weeks [1]. An underdose may also present as an escalation of the primary pain complaint or withdrawal symptoms related to the pump medications [1]. However, if high concentrations of drug are used, symptoms of overdose usually occur rapidly. For instance, 40 mL of morphine 20 mg/mL would result in a systemic dose of 800 mg, which can be lethal if appropriate life support measures are not in place.
In data collected from May 1996 to September 2010, Medtronic received 351 reports worldwide related to occurrence of pocket fills with their intrathecal infusion pumps [1]. Assuming pumps are refilled six times per year on average, the reported rate of occurrence per refill opportunity is about 0.01% although the actual occurrence rate is likely higher due to underreporting [1]. Of the reported events, there have been 8 deaths, 270 events requiring medical intervention, and 58 events not requiring medical intervention [1]. There were 15 events in which the patient severity was unknown [1].
If a pocket fill occurs and is recognized, the pump pocket should be accessed with a large bore needle and attempts made to remove as much of the contents as possible. If available, ultrasound can be used to identify the fluid pocket to aspirate. The patient should be monitored for signs and symptoms of medication overdose in an appropriate facility for a reasonable amount of time or until symptoms have resolved. If a pocket fill is suspected, it may be useful to empty the pump reservoir completely and compare the volume removed to the expected volume. A discrepancy may indicate that a pocket fill has occurred [1]. Swelling at the injection site or patient report of an unusual sensation during drug injection such as pressure, stinging, or burning may also indicate the presence of a pocket fill although their absence do not rule out the occurrence of a pocket fill [1].
32.6 Pump Stall
Although a spontaneous pump stall is possible, the most common time for a pump to stall is during an MRI. Medtronic intrathecal drug delivery systems are driven by a peristaltic rotor. The rotor will stop due to the magnetic fields of the MRI scanner [2]. The pump should restart spontaneously after MRI has ended, but a permanent stall is possible. There are other pumps on the market with different mechanisms less susceptible to an MRI-related pump stall, but the number in service is substantially smaller than for the Medtronic systems [2]. Medtronic recommends that a pump should be interrogated after an MRI to determine whether the pump has resumed function [2]. The event log should show messages indicating that a motor stall and subsequent motor recovery have occurred [2]. If a recovery has not occurred, the patient is at risk for a withdrawal syndrome related to intrathecal medications, especially baclofen [2]. If a stall is identified, wait another 20 min and interrogate the pump again to address delays in event logging due to electromagnetic interference from the MRI [2]. The device representative should be contacted if the pump has not restarted at this point, and care should be taken to prevent withdrawal symptoms [2]. More information regarding pump stall can be found in the chapter entitled, “Intrathecal Pump Malfunction: Stall, Flip, Expired.”
32.7 Pump Programming Error
Errors in intrathecal pump programming can occur which could result in either overdose or underdose. This may be due to incorrectly programming the concentration of the medications placed into the pump. Programming errors can also occur when entering the daily infusion dose of medication to be delivered. Changes from the previous pump settings will result in a warning if the programmed dose to be delivered is either higher or lower. Rechecking the intended settings and comparing them to doses and concentrations recorded in the patient’s chart will quickly determine if this is a potential cause of withdrawal.
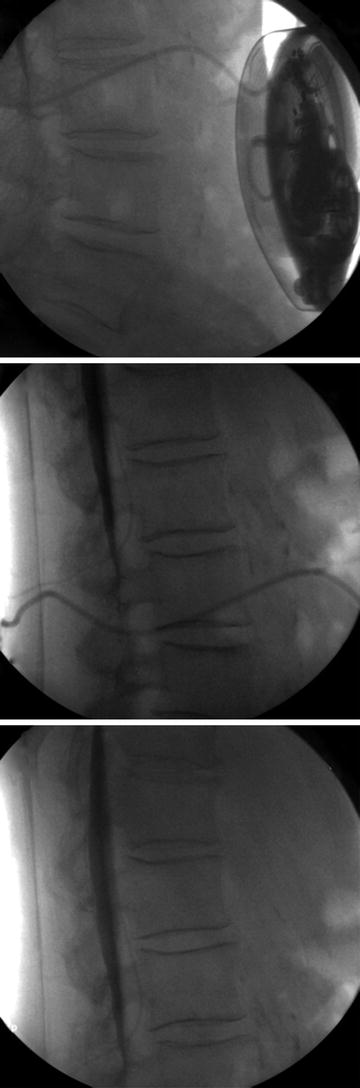
32.8 Catheter Fracture/Leak
Catheter fracture or leak may occur due to trauma. Trauma can occur with pulling or sheering of the catheter, repetitive motion damage, pump flip in the pocket, or needle trauma to the catheter during refill or side port aspiration. Such damage may present clinically as worsening of the primary pain complaint, cerebrospinal fluid leak including symptoms of low-pressure CSF headache, or withdrawal from intrathecal pump medications. Medtronic followed intrathecal catheters from 2003 to 2014 via a registry. Of the 7154 catheters followed in the registry, 161 catheters showed evidence of a break or a cut [3].
The first step in assessing for a catheter complication is to aspirate the side port of the intrathecal pump. If CSF can be freely aspirated, then contrast may be injected under fluoroscopy. Never inject through the side port if CSF cannot be freely aspirated. To do so may result in bolus intrathecal administration of residual drug in the catheter. Tracing contrast injected through the catheter from the pump into the intrathecal space may identify a leak of contrast or failure to deliver contrast to the subarachnoid space [4] (Fig. 32.2).