CHAPTER 38 Inotropic and Vasoactive Agents in the Cardiac Intensive Care Unit
These agents are used to correct or stabilize hemodynamic function and, therefore, in many cases their proper selection and dosing requires hemodynamic information based on a pulmonary artery catheter, an intra-arterial pressure monitor, and electrocardiographic monitoring (Table 38-1). However, the use of a pulmonary artery catheter did not show benefit in either acute decompensated heart failure patients in the ESCAPE trial1 or a recent meta-analysis of 13 randomized trials including more than 5000 critically ill patients.2 This lack of benefit may result from the absence of effective strategies to use in combination with pulmonary artery catheter information. These studies also demonstrated no increase in mortality or hospitalization associated with pulmonary artery catheter use. Based on the available data, there is no indication for routine use of a pulmonary artery catheter in patients hospitalized with acute decompensated heart failure. Nevertheless, a pulmonary artery catheter may provide valuable information and help guide therapy in specific situations (e.g., patients in whom congestion does not resolve after initial therapy, or in whom the presence of congestion is unclear or associated with worsening renal function).
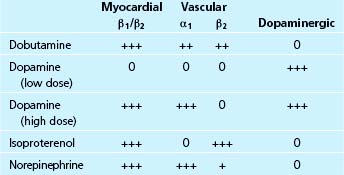
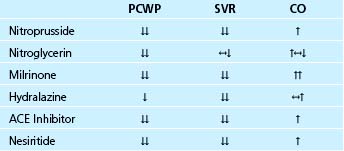
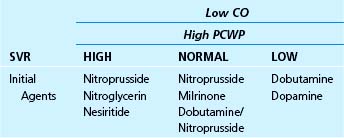
Table 38–1 Intravenous Drug Selection in Patients with Elevated Left Heart Filling Pressures and a Reduced Cardiac Output
Sympathomimetic Agents
Dopamine
Dopamine is the immediate precursor of epinephrine and norepinephrine. It has both cardiac and vascular sites of action, depending in part on the dose used3,4 (Table 38-2). At low doses (i.e., 1 to 3 μg/kg/min), dopamine directly activates dopaminergic receptors in the kidney and splanchnic arteries, thereby causing vasodilation of these beds. The resultant increase in renal blood flow leads to increased urine output and sodium excretion. At moderate doses (i.e., 3 to 8 μg/kg/min), dopamine is a weak partial agonist at myocardial β1-receptors and causes the release of norepinephrine from sympathetic nerve terminals in the myocardium and vasculature. The direct stimulation of myocardial β-adrenergic receptors exerts positive chronotropic and inotropic effects. The increased release of norepinephrine from nerve terminals (a tyramine-like effect) also contributes to myocardial stimulation, but in addition may exert a mild vasoconstrictor effect due to stimulation of vascular α-adrenergic receptors. At high doses of dopamine (i.e., 5 to 20 μg/kg/min), the effect of peripheral α-adrenergic stimulation predominates, resulting in vasoconstriction in all vascular beds and leading to increases in mean arterial pressure and systemic vascular resistance. At high doses, the vasoconstrictor effect overshadows the dopaminergic vasodilator effects, so that renal blood flow decreases and urine output may decline. However, in patients with acute decompensated heart failure the dose required for improving systemic and renal hemodynamics may be higher (on the order of 4 to 6 μg/kg/min) than the usual “low dose” range, leading to the suggestion that severe heart failure may impair the renal effects of dopamine.5
Given its varying actions, there are several potential uses for dopamine in the cardiac intensive care unit. In patients with decompensated heart failure, dopamine is frequently used at low infusion rates to improve renal function by increasing renal blood flow.6,7 Increased water and sodium excretion results in a decrease in right and left ventricular filling pressures. Low dose dopamine is frequently combined with one or more other inotropic (e.g., dobutamine) or vasodilator (e.g., nitroprusside) agents.8
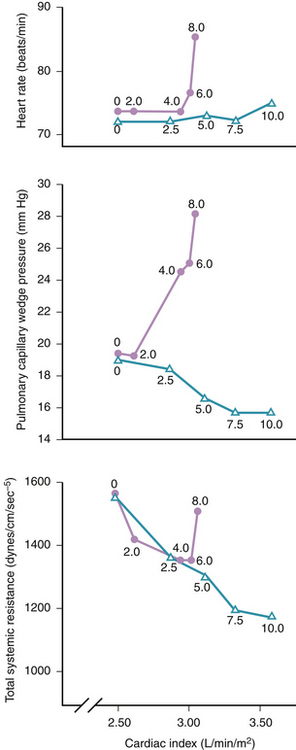
In patients with severe compromise of the arterial pressure or frank cardiogenic shock, higher doses of dopamine are used to increase systemic vascular resistance. At these higher doses, the increased left ventricular afterload is partially offset by the positive inotropic action. In addition, when it is necessary to use vasoconstrictor doses of dopamine to manage systemic hypotension in the setting of myocardial failure, it is often useful to add dobutamine to augment the level of positive inotropic support beyond that provided by dopamine alone. When used alone at vasoconstrictor doses in patients with left ventricular failure, dopamine may increase both left and right heart filling pressures9 (Fig. 38-1 and Table 38-3). This effect reflects increased left and right ventricular afterload and increased peripheral venoconstriction, the latter causing increased return of venous blood to the heart. To counteract these actions, high dose dopamine is sometimes combined with vasodilators (e.g., nitroglycerin).10
The inotropic responses to dopamine may be attenuated because of desensitization of the β-adrenergic pathway and depletion of myocardial catecholamine stores, both of which are common in patients with advanced heart failure.11,12 Although generally well tolerated at low doses, higher infusion rates of dopamine may result in unwanted sinus tachycardia and/or arrhythmias (supraventricular and ventricular). Other adverse effects of dopamine include digital gangrene in patients with underlying peripheral arterial disease, tissue necrosis at sites of infiltration, and nausea at high doses. Local infiltration may be counteracted by the local injection of the α-adrenergic antagonist phentolamine.
Dobutamine
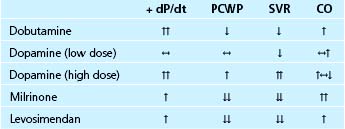
Dobutamine is a direct-acting synthetic sympathomimetic amine that stimulates β1-, β2-, and α-adrenergic receptors (see Table 38-2). Clinically, it is available as a racemic mixture in which the (+) enantiomer is both a β1– and β2-adrenergic receptor agonist and an α-adrenergic receptor competitive antagonist, and the (−) enantiomer is a potent β1-adrenergic receptor agonist and an α-adrenergic receptor partial agonist.13,14 The net effect of this pharmacologic profile is that dobutamine causes a relatively selective stimulation of β1-adrenergic receptors, and accordingly, dobutamine’s primary cardiovascular effect is to increase cardiac output by increasing myocardial contractility. This positive inotropic effect is associated with relatively little increase in heart rate. The drug causes modest decreases in left ventricular filling pressure and systemic vascular resistance due to a combination of direct vascular effects and the withdrawal of sympathetic tone15 (see Table 38-3). Dobutamine also directly improves left ventricular relaxation (positive lusitropic effect) via stimulation of myocardial β-adrenergic receptors.16 Dobutamine has no effect on dopaminergic receptors and therefore no direct renal vasodilator effect. However, renal blood flow often increases with dobutamine in proportion to the increase in cardiac output.
Dobutamine is a valuable agent for the initial management of patients with acute or chronic systolic heart failure characterized by a low cardiac output.17 It is often initiated at an infusion rate of 2 μg/kg/min (without a loading dose) and titrated upward by 1 to 2 μg/kg/min every 15 to 30 minutes until the hemodynamic goal is reached or a dose-limiting event, such as unacceptable tachycardia or arrhythmias, occurs. Maximum effects are usually achieved at a dose of 10 to 15 μg/kg/min, although higher infusion rates may occasionally be used. In patients with more severe decompensation, and presumably greater β-adrenergic receptor downregulation, dobutamine can be started at 5 μg/kg/min. If the maximum tolerated infusion rate of dobutamine does not result in a sufficient increase in cardiac index, a second drug (e.g., milrinone) may be added.8,18 In patients with elevated systemic vascular resistance and/or left heart filling pressures, the co-administration of a vasodilator such as nitroprusside or nitroglycerin may be required. In patients who remain hypotensive on dobutamine, consideration should be given to the addition of a pressor dose of dopamine and/or the use of mechanical circulatory support.
Other clinical situations in which dobutamine is effective include cardiogenic shock complicating acute myocardial infarction, low cardiac output following cardiopulmonary bypass, and as a “bridge” to cardiac transplantation.19 There is some evidence that short-term or intermittent infusions of dobutamine can result in sustained improvement in hemodynamics and functional status for days or weeks after the infusion is stopped.20–22 However, there are limited clinical data to suggest that the intermittent use of dobutamine either has no effect on outcomes23 or may increase mortality.24 As a result, the administration of dobutamine should be limited to the inpatient setting.
Dobutamine may increase heart rate, thereby limiting the dose that can be infused. However, in some patients with very depressed cardiac output the improvement in hemodynamic function may cause a withdrawal of sympathetic tone such that heart rate falls. Hypotension is uncommon, but can occur in patients who are hypovolemic. Arrhythmias, including supraventricular and ventricular tachycardia, may limit the dose. Likewise, myocardial ischemia secondary to increased myocardial oxygen consumption may occur. Some patients with chronic severe heart failure may be tolerant to dobutamine, or tolerance to dobutamine may develop after several days of a continuous infusion.25 In this situation, the addition or substitution of a phosphodiesterase inhibitor may be helpful. Hypersensitivity myocarditis has also been reported with chronic infusions of dobutamine and should be suspected if a patient develops worsening hemodynamics or peripheral eosinophilia.
Isoproterenol
A synthetic sympathomimetic structurally related to epinephrine, isoproterenol is a nonselective β-adrenergic receptor agonist with little or no effect on α-receptors (see Table 38-2). Its cardiovascular effects include increased myocardial contractility, heart rate, and atrioventricular conduction due to stimulation of myocardial β1– and β2-adrenergic receptors, and vasodilation of skeletal muscle and pulmonary vasculature due to stimulation of vascular β2-adrenergic receptors. Isoproterenol increases cardiac output and lowers both systemic and pulmonary vascular resistance.
Because of its propensity to increase heart rate, isoproterenol has relatively limited applications in the cardiac intensive care unit. However, isoproterenol may be useful in the management of torsades de pointes that is refractory to magnesium,26 inotropic and chronotropic support immediately following cardiac transplant,27 and treatment of pulmonary hypertension secondary to acute pulmonary embolism.28 Isoproterenol is usually administered as a continuous infusion at 0.5 to 5 μg/min. The dose of isoproterenol may be limited by tachycardia, increased myocardial oxygen consumption leading to ischemia, and atrial or ventricular arrhythmias.
Epinephrine
Like isoproterenol, epinephrine stimulates β1– and β2-adrenergic receptors in the myocardium, thereby causing marked positive chronotropic and inotropic responses. Unlike isoproterenol, it also has potent agonist effects at vascular α-adrenergic receptors causing increased arterial and venous constriction. Because of this latter effect, epinephrine (like high-dose dopamine and norepinephrine) plays little role in the acute management of heart failure, except when complicated by severe hypotension. Epinephrine may be useful for the treatment of low cardiac output, with or without bradycardia, immediately following cardiopulmonary bypass or cardiac transplantation.29 Continuous infusions may be started at a low dose (0.5 to 1 μg/min), and titrated upwards to 10 μg/min, as needed. The use of epinephrine may be limited by tachycardia, arrhythmias, increased myocardial oxygen consumption leading to ischemia, and oliguria from renal vasoconstriction.
In the setting of cardiac arrest, epinephrine may be used as per the Advanced Cardiac Life Support (ACLS) protocol (1 mg intravenous push or via endotracheal tube every 3 to 5 minutes) to manage ventricular fibrillation, pulseless ventricular tachycardia, asystole, or pulseless electrical activity.30 Epinephrine may also be infused at 2 to 10 μg/min to manage symptomatic bradycardia that is unresponsive to atropine, while awaiting placement of an external or temporary transvenous pacemaker.
Norepinephrine
The myocardial and peripheral vascular effects of this endogenous catecholamine are similar to those of epinephrine except that norepinephrine causes little stimulation of vascular β2–adrenergic receptors and therefore causes more intense vasoconstriction (see Table 38-2). Norepinephrine may be used to provide temporary circulatory support in the setting of hemodynamically significant hypotension (e.g., following cardiac surgery or with cardiogenic shock complicating acute myocardial infarction or pulmonary embolism). Norepinephrine is titrated to improve blood pressure at doses of 2 to 10 μg/min. As with epinephrine, the use of norepinephrine in the cardiac intensive care unit may be limited by arrhythmias, myocardial ischemia, renal impairment, or tissue necrosis at the site of local infiltration. If extravasation occurs, phentolamine 5 to 10 mg may be infiltrated into the affected area.
Phosphodiesterase Inhibitors
The breakdown of cAMP is mediated by a membrane-bound enzyme, phosphodiesterase (PDE). In myocardium and vascular smooth muscle, the predominant isoform of this enzyme, termed type III, is inhibited by the type-III selective PDE inhibitors milrinone and inamrinone, leading to an increase in intracellular cAMP concentrations. In the myocardium, intracellular cAMP increases both contractility and the rate of relaxation (positive lusitropic effect). PDE III inhibitors are also potent vasodilators in the systemic and pulmonary vasculature.31–33
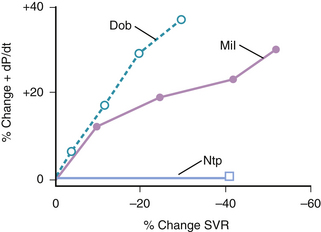
In patients with acute decompensated heart failure, type III PDE inhibitors increase cardiac output by increasing stroke volume. Balanced arterial and venous dilation cause decreases in right atrial, pulmonary artery, pulmonary capillary wedge, and mean arterial pressures. Because PDE inhibitors exert both positive inotropic and vasodilator actions, their net hemodynamic effects differ from those of dobutamine and nitroprusside. Thus, for a comparable increase in cardiac output, the PDE inhibitor milrinone decreases systemic vascular resistance and left ventricular filling pressure to a greater extent than dobutamine34 (Fig. 38-2; see Table 38-3). Conversely, for a comparable decrease in arterial blood pressure, milrinone increases cardiac output to a greater extent than nitroprusside35 (Table 38-4).
PDE inhibitors are used for the treatment of heart failure characterized by low cardiac output, high filling pressures, and elevated or normal systemic vascular resistance. They may also be useful in the management of low cardiac output following cardiopulmonary bypass and as a bridge to mechanical support or cardiac transplant,36
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree