Injections for Headache (Occipital Nerve Blocks and Botulinum Toxin Injections)
The medical equivalent of epic novels has been written on the comprehensive management of headache. Per the mission of this book, only the basics of two commonly performed procedures used to treat headache will be discussed. These procedures are occipital nerve blocks (ONBs) and botulinum toxin injections. The goal is to add two main procedures to your armamentarium of treating occipital neuralgia and headache.
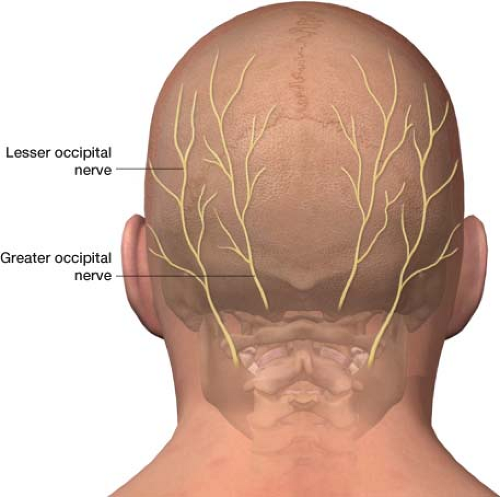
The greater occipital nerve is composed of sensory fibers that primarily originate from the C2 nerve root and to a lesser extent the C3 nerve root. The greater occipital nerve penetrates the nuchal fascia at the base of the skull. It tracks cephalad to innervate the medial portion of the posterior scalp (Fig. 26-1). The lesser occipital nerve also arises from a branch of the C2 nerve root or may originate from a combination of the
C2 and C3 nerve roots. It innervates the lateral portion of the posterior scalp and the pinna of the ear. Classically, the description of occipital nerve pain, occipital neuralgia, is a deep or burning pain with shock-like features over the occipital region. Injury to the occipital nerves can occur anywhere along their course from the cervical spinal roots to the tip of the nerve endings.
C2 and C3 nerve roots. It innervates the lateral portion of the posterior scalp and the pinna of the ear. Classically, the description of occipital nerve pain, occipital neuralgia, is a deep or burning pain with shock-like features over the occipital region. Injury to the occipital nerves can occur anywhere along their course from the cervical spinal roots to the tip of the nerve endings.
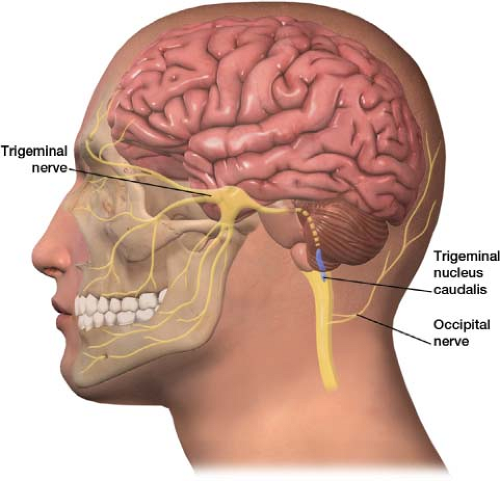
The trigeminal nucleus (origin of the trigeminal nerve) extends from the brainstem down to the upper cervical cord, where it comes into close approximation with the dorsal horns of the C1 and C2 nerve roots (remember, the occipital nerves originate from the C2 nerve root). This anatomic relationship is known as the trigeminal cervical complex (TCC) (Fig. 26-2). The TCC is thought to receive impulses from both the first division of the trigeminal nerve and nociceptive impulses from the greater occipital nerve.1–5 Synaptic overlay at the trigeminocervical nucleus may allow for the referral of pain from the occipital nerve to any of the branches of the trigeminal nerve, with a propensity for the retro-orbital region.5 This is a referred pain. Stimulation of the greater occipital nerve increases excitability of the dural connection to the trigeminocervical nucleus.2 This explains how occipital neuralgia can cause pain that reaches beyond the classical distribution of the occipital nerve. It may be that stimulation of the occipital nerve can cause headache mimicking that of a migraine.
Migraine is an episodic neurologic disorder that affects roughly 17% of women and 6% of men. The pain of migraine is thought to be mediated by trigeminal nerve afferents, which provide sensory innervation to the face and intracranial structures such as the dura and the cerebral vasculature (remember, brain tissue has no pain receptors). Although the exact cause is unknown, current hypotheses suggest that activation of the trigeminal nerve triggers a cascade of changes to the brain’s dura and vasculature that leads to producing the migraine.
Research in humans has shown that an ONB, while effective for pain along the posterior scalp, may also lead to pain relief outside of the skin territory supplied by the nerve.6,7 Again, ONBs temporarily reduce afferent nociceptive impulses to the trigeminocervical nucleus. This aberrant stimulation of trigeminal nerve innervated structures ceases, breaking the pain cycle. It has been hypothesized that an ONB leads to a “wind down” of central sensitization of migraine. ONBs are
also very beneficial for isolated occipital neuralgia when the direct skin territory supplied by the nerve is affected. Often palpating the nerve in the occipital groove elicits the pain. Open-label studies have found that, ONBs are potentially effective for the treatment of migraine, cluster headache, occipital neuralgia, and post-traumatic headache.
also very beneficial for isolated occipital neuralgia when the direct skin territory supplied by the nerve is affected. Often palpating the nerve in the occipital groove elicits the pain. Open-label studies have found that, ONBs are potentially effective for the treatment of migraine, cluster headache, occipital neuralgia, and post-traumatic headache.
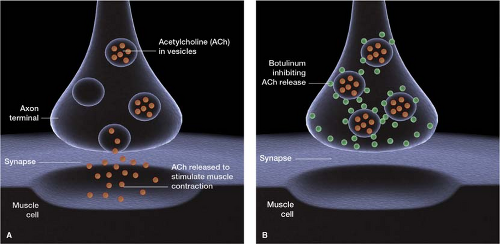
Dermatologists and plastic surgeons using botulinum toxin type A (Botox) to minimize hyperfunctional facial lines first reported that it prevented migraine headache. A number of patients receiving the injection for hyperfunctional facial lines indicated that it was also very helpful for their headaches, suggesting a possible association. These observations eventually led to a preliminary open-label study, which concluded that botulinum toxin is a beneficial therapeutic agent for both the acute and prophylactic treatment of migraine.8 The toxin works by inhibiting the vesicular release of the neurotransmitter acetylcholine (ACh) at the neuromuscular junction, leading to muscle paresis or paralysis (Fig. 26-3).
Investigators originally theorized that botulinum toxin injections helped migraines by relieving muscle spasm and tension, which may be a trigger. A more contemporary understanding of migraine pathophysiology challenges this initial theory. The later theory refutes “muscle tension” as a likely trigger for migraine and observes that the prophylaxis of headache pain appears to outlast the paralysis of muscle in many patients. Also, some patients have reported that botulinum toxin is effective even when injected into areas not supplied by muscle (such as on the apex of the head). In addition, botulinum toxin is beneficial for migraine in acute episodes, taking effect within 1 to 2 hours, whereas muscle paralysis does not take hold for 3 days. Despite reports of improvement during acute attacks, botulinum toxin injections have a more robust body of evidence supporting their use as a prophylactic agent that sometimes improves over subsequent injections. Although the true mechanism by which botulinum toxin injections are effective for migraine prophylaxis is unclear, theories have suggested that they may exert a direct antinociceptive sensory block on trigeminal triggers of migraines.9
Additional mechanisms of action for botulinum toxin may involve antivasodilatory and anti-inflammatory properties. The toxin inhibits the release of nociceptive mediators, such as calcitonin gene-related peptide, substance P, and glutamate from peripheral termini of primary afferent nerves. By blocking the release of these neurotransmitters, the toxin blocks neurogenic inflammation, which in turn inhibits peripheral pain signals to the central nervous system. Clinical studies have reinforced its effectiveness. In a large randomized, double-blind, placebo-controlled trial of botulinum toxin A (PREEMPT), 1,384 patients with chronic migraine showed a significant reduction in headache days, improvement in functioning, and less disability as compared with placebo.9
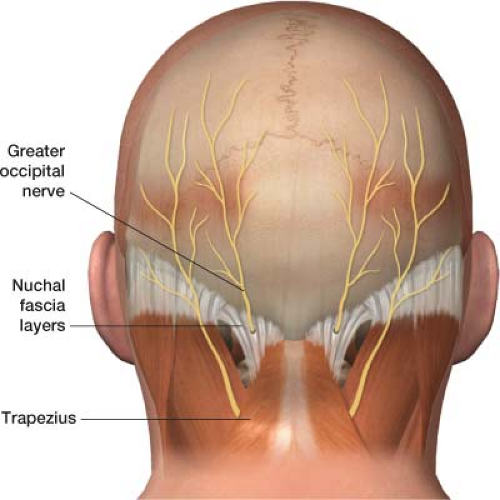 Figure 26-4 The greater occipital nerve lies at the base of the skull between the nuchal fascia layers and the trapezius muscle. The nerve is prone to flexion/extension injuries, as well as entrapment by spasm of the trapezius.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|