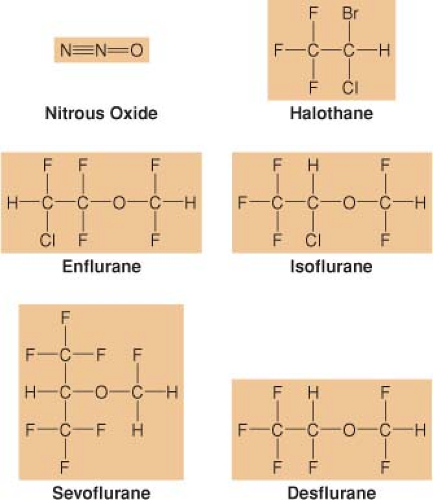
Inhaled Anesthetics
The popularity of inhaled anesthetics for establishing general anesthesia is based on their ease of administration (via inhalation) and the ability to monitor their effects (clinical signs and end-tidal concentrations) (Fig. 17-1) (Ebert TJ, Linderman L. Inhaled anesthetics. In: Barash PG, Cullen BF, Stoelting RK, Cahalan MK, Ortega R, Stock MC, eds. Clinical Anesthesia. Philadelphia: Lippincott Williams & Wilkins; 2013:445–477). The most popular potent inhaled anesthetics used in adult surgical procedures are sevoflurane, desflurane, and isoflurane (see Fig. 17-1). Sevoflurane is the most commonly used inhaled anesthetic for pediatric patients.
I. Pharmacokinetic Principles
Drug pharmacology is classically divided into pharmacodynamics (what the body does to a drug) and pharmacokinetics (what the drug does to the body). Drug pharmacokinetics has four phases: absorption (uptake), distribution, metabolism, and excretion (elimination).
Unique Features of Inhaled Anesthetics
Speed, Gas State, and Route of Administration. The inhaled anesthetics are among the most rapidly acting drugs in existence, and when administering a general anesthetic, this speed provides a margin of safety and also means efficiency.
Technically, nitrous oxide and xenon are the only true gases; the other inhaled anesthetics are vapors of volatile liquids (for simplicity, all of them are referred to as gases).
A unique advantage of anesthetic gases is the ability to deliver them to the bloodstream via the patient’s lungs.
The goal of delivering inhaled anesthetics is to produce the anesthetic state by establishing a specific concentration (partial pressure) in the central nervous system (CNS). This is achieved by establishing the desired partial pressure in the lungs that ultimately equilibrates with the brain and spinal cord.
At equilibrium, the CNS partial pressure equals the blood partial pressure, which equals alveolar partial pressure.
Gases in Mixtures. For any mixture of gases in a closed container, each gas exerts a pressure proportional to its fractional mass (partial pressure).
Gases in Solutions
The concentration of any one gas in a mixture of gases in solution depends on (i) its partial pressure in the gas phase in equilibrium with the solution and (ii) its solubility within that solution.
The concentration of anesthetic in the target tissue depends on the partial pressure at equilibrium and the target tissue solubility.
Because inhaled anesthetics are gases and because partial pressures of gases equilibrate throughout a system, monitoring the alveolar concentration of inhaled anesthetics provides an index of their effects on the brain.
Anesthetic Transfer: Machine to Central Nervous System (Table 17-3)
Uptake and Distribution
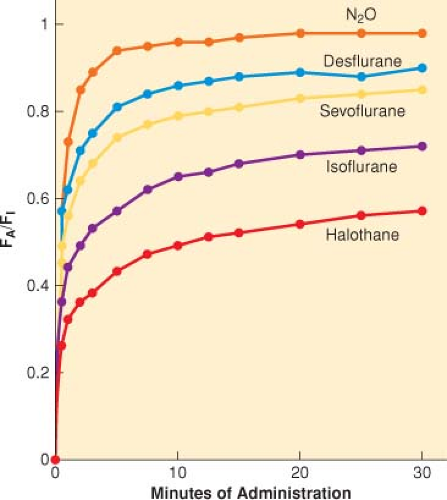
FA/FI. A common way to assess anesthetic uptake is to follow the ratio of the alveolar anesthetic concentration (FA) to the inspired anesthetic concentration (FI) over time (FA/FI) (Fig. 17-2).
Distribution (Tissue Uptake). Factors that increase or decrease the rate of increase of FA/FI determine the speed of induction of anesthesia (see Fig. 17-2 and Table 17-3).
Metabolism plays little role in opposing induction but may have some significance in determining the rate of recovery.
Overpressurization and Concentration Effect
Overpressurization (delivering a higher FI than the FA actually desired for the patient) is analogous to an intravenous bolus and thus speeds the induction of anesthesia.
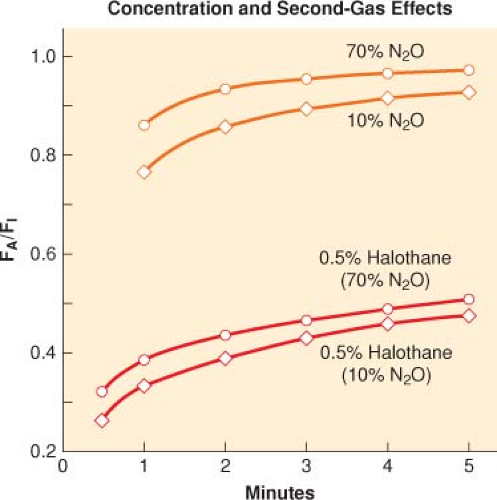
Concentration effect (the greater the FI of an inhaled anesthetic, the more rapid the rate of increase of the FA/FI) is a method used to speed the induction of anesthesia (Fig. 17-3).
Second Gas Effect
A special case of the concentration effect is administration of two gases simultaneously (nitrous oxide and a potent volatile anesthetic) in which the high volume uptake of nitrous oxide increases the FA (concentrates) of the volatile anesthetic.
Ventilation Effects
Inhaled anesthetics with low blood solubility have a rapid rate of increase in the FA/FI with induction of anesthesia such that there is little room to improve this rate of increase by increasing or decreasing ventilation (see Fig. 17-3).
To the extent that inhaled anesthetics depress ventilation with an increasing FI, alveolar ventilation decreases, as does the rate of increase of FA/FI (negative feedback that results in apnea and may prevent an overdose).
Perfusion Effects
As with ventilation, cardiac output does not greatly affect the rate of increase of the FA/FI for poorly soluble anesthetics.
Cardiovascular depression caused by a high FI results in depression of anesthetic uptake from the lungs and increases the rate of increase of FA/FI (positive feedback that may result in profound cardiovascular depression).
Exhalation and Recovery
Recovery from anesthesia, similar to induction of anesthesia, depends on the drug’s solubility (primary
determinant of the rate of decrease in FA), ventilation, and cardiac output (Fig. 17-4).
The “reservoir” of anesthetic in the body at the conclusion of anesthesia is determined by the solubility of the inhaled anesthetic and the dose and duration of the drug’s administration (can slow the rate of decrease in the FA).
Pharmacokinetic differences between recovery and induction of anesthesia include the absence of overpressurization (cannot give less than zero) during recovery and the presence of tissue anesthetic concentrations present at the start of recovery (tissue concentration of zero at the start of anesthesia induction).
Table 17-1 Physiochemical Properties of Volatile Anesthetics | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 17-2 Tissue Groups and Pharmacokinetics | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Table 17-3 Factors That Increase or Decrease the Rate of Increase of Alveolar Anesthetic Concentration (FA)/Inspired Anesthetic Concentration (FI) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|