Independent Lung Ventilation: Introduction
Independent lung ventilation (ILV) was first used in thoracic surgery and the intubation devices were developed for this purpose. Gale and Waters first reported ILV in 1931, by passing a single-lumen endobronchial tube into the main bronchus of the dependent nonoperative lung for ventilation and exclusion of purulent secretion (if present) from the operative lung. In 1936, Magill1 reported endobronchial placement of a suction catheter with a balloon to occlude the operative bronchus and tracheal placement of an ETT to ventilate the nonoperative lung. In 1947, Moody2 encased the endobronchial balloon with metal studs to reduce the risk of balloon dislodgment.
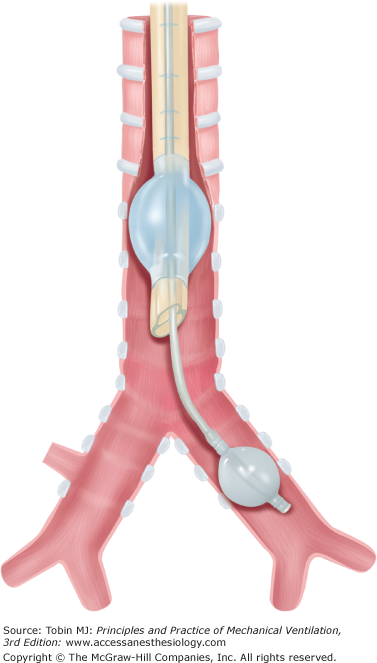
The first double-lumen tube (DLT), enabling independent ventilation of both lungs, was reported by Carlens3 in 1949. Thus, tube was similar to current left DLTs, but had a rubber “hook” to engage the carina for accurate placement. Although a major advance, this tube caused trauma and was unsuitable for left pneumonectomy. The first right DLT, which did not occlude the right upper lobe bronchus, was not reported until 1960 by White.4 In 1962, Robertshaw5 reported right and left DLTs, which served as the prototype of today’s DLTs. Current DLTs have replaced red rubber with polyvinyl chloride (PVC) to reduce mucosal injury and improve malleability and airflow (Fig. 25-1).
For many years, DLTs and ILV were used entirely for thoracic surgery.2,5,6 In 1976, Glass and Trew7,8 and their coworkers reported ILV for nonsurgical purposes: respiratory insufficiency from unilateral lung disease. Since then, application of ILV has broadened to a wide range of conditions (Table 25-1) employing a variety of techniques. Institutions specializing in conditions commonly requiring ILV (such as single lung transplantation or alveolar proteinosis), ILV may be used in approximately 0.5% of all mechanically ventilated patients.9,10 Although most intensive care units use ILV in fewer than one in 1000 patients requiring mechanical ventilation, it can be a lifesaving measure in specific conditions, making maintenance of suitable equipment and knowledge of its use required.
| Size (French) | OD (mm) | Tracheal ID (mm) | Bronchial ID (mm) | Use | Manufacturera |
|---|---|---|---|---|---|
| 26 | 8.7 | 3.5 | 3.5 | Rüsch | |
| 28 | 9.3 | 3.1 | 3.2 | Children weighing <40 kg | Mallinckrodt |
| 32 | 10.7 | 3.5 | 3.4 | Sheridan | |
| 35 | 11.7 | 4.5 | 4.3 | Children weighing >40 kg | Mallinckrodt |
| 37 | 12.3 | 4.7 | 4.5 | Small adult | Mallinckrodt |
| 39 | 13.0 | 4.9 | 4.9 | Medium adults, usual female size | Mallinckrodt |
| 41 | 13.7 | 5.4 | 5.4 | Large adult, usual male size | Mallinckrodt |
ILV is infrequently and often urgently required when conventional mechanical ventilation is not a viable alternative. As a result, there have been no randomized controlled trials of ILV in patients and current evidence is based almost entirely on animal models, case reports, and case series with before and after analyses.
Basic Principles of Independent Lung Ventilation
The need to protect one lung from harmful effects of fluid in the other lung (blood, purulent or malignant secretions, lavage fluid). Placing the fluid-filled lung in the dependent position (lateral decubitus) minimizes the risk of unwanted fluid entering the other lung but also maximizes hypoxia11 by maximizing blood diversion to the less-functional lung. Placing the fluid-filled lung in the nondependent position improves oxygen saturation (SaO2), but the risk from fluid spillage in this position is unacceptably high.11 The supine position is usually the best compromise, except during thoracic surgery, where the operative lung must be in the uppermost position.
The need to isolate the ventilatory patterns to each lung. In each case, the ventilatory pattern for one lung has disadvantageous effects if applied to both lungs. This includes one-lung ventilation (OLV; e.g., thoracic surgery, whole-lung lavage), ventilation to each lung differing in only a single variable (e.g., positive end-expiratory pressure [PEEP]), or ventilation requirements differing in every variable (e.g., single-lung transplant for obstructive airways diseases), or the need to prevent air loss from one lung (e.g., massive air leak).
If OLV is required, a bronchial blocker and endobronchial tube or a DLT may be used. If ILV is required, then a DLT is usually used, although some bronchial blockers can allow limited ventilation such as continuous positive airway pressure (CPAP) or jet ventilation.
Independent Lung Ventilation Techniques
ILV must be preceded by the placement of a DLT or alternate lung isolation device. These must be introduced, correctly positioned, and then tested to ensure isolation of lung ventilation.
Compared with red-rubber DLTs,3,4,12 PVC-type DLTs (see Fig. 25-1, e.g., Mallinckrodt, Sheridan, Rusch, Concord, Portex, Marraro) are more flexible, have better internal–external diameter10 ratios, better gas-flow characteristics, easier suction and bronchoscopy access,13 allow airway seal with lower cuff pressures,14 are less irritating to respiratory mucosa, have a lower risk of trauma, and are easier and quicker to position.15 These characteristics make PVC types the DLTs of choice, although airway injury from PVC DLTs may still occur.16,17
For most indications, a left DLT (see Fig. 25-1) should be used because placement is easier than for a right DLT, which has a high risk of right upper-lobe occlusion.18 A right DLT (see Fig. 25-1) is required for thoracic surgical procedures that include the left main bronchus (left pneumonectomy, left main bronchial lesions, stenosis, or rupture), thoracic aortic aneurysm repair, or anatomic abnormalities that prevent satisfactory access to the left main bronchus.19–21 A right DLT is also required for ILV with a recent left single lung transplantation to avoid injury to the anastomosis and distal airway. To minimize airflow resistance and maximize endobronchial access, the largest DLT that will not cause laryngeal or airway injury should be chosen (see Table 25-1).
PVC DLTs are usually introduced with the endobronchial curvature angled anteriorly and a rigid stylet in situ to facilitate the passage of the tip through the vocal cords. Once through the cords, the stylet is usually removed and the DLT is rotated through 90 degrees so that the endobronchial curvature is directed toward the appropriate side. The tube is then advanced until an increase in resistance is detected. In a randomized trial of sixty patients receiving a DLT for thoracic surgery, Lieberman et al22 compared removal of the stylet (as above) and leaving the stylet in situ until placement was complete; the latter increased the correct placement rate from 17% to 60%.
DLT tube position and function must then be confirmed by one or more of three techniques:
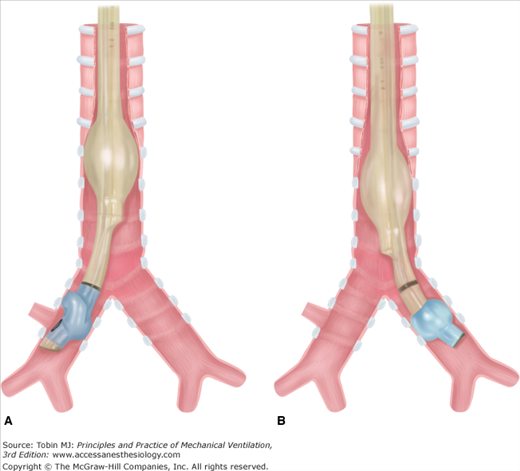
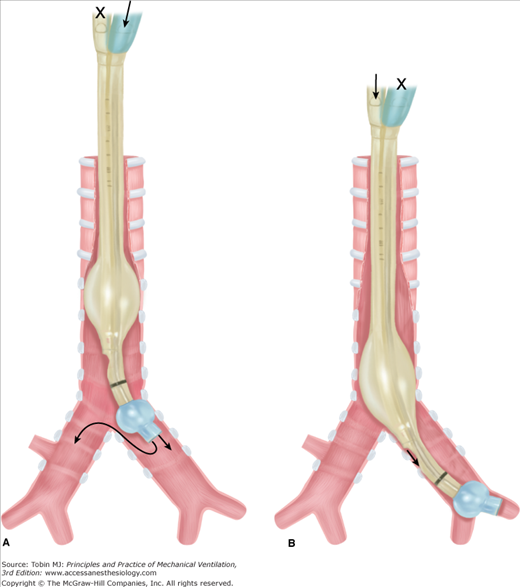
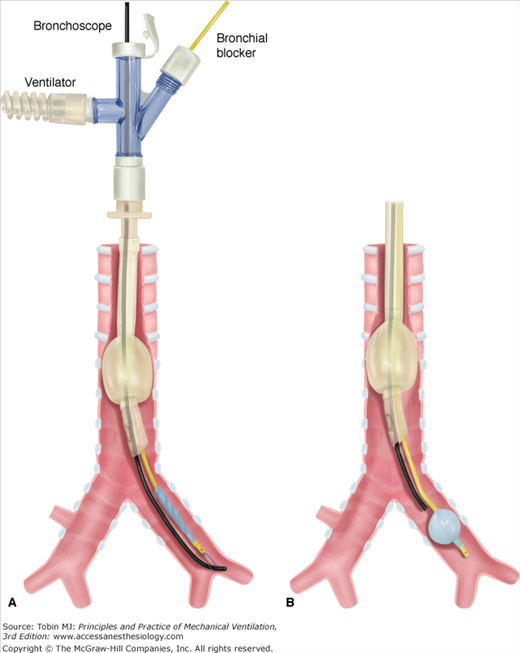
Auscultation. Following cuff inflation, the tracheal port should be clamped and the bronchial port ventilated.19 Bilateral or contralateral breath sounds indicate placement is too proximal and a need to reposition the DLT (Fig. 25-2A), whereas breath sounds heard only on the correct side indicate correct placement (see Fig. 25-1). Difficulty with ventilation should be resolved by deflating the bronchial cuff: bilateral breath sounds indicate that placement is too proximal, whereas breath sounds heard only on the side of the endobronchial tube indicate that placement is too distal (Fig. 25-2B). Lung auscultation should always include both upper and lower lobes, to ensure correct placement of the endobronchial port within the bronchus, especially with a right DLT where the right upper lobe is easily occluded.
Bronchoscopy. Following DLT insertion, a small fiberoptic bronchoscope may be passed through the tracheal lumen to confirm position of the tracheal port and that the endobronchial tube is in the correct position.19,23 The endobronchial cuff should be visible just distal to the carina. Subsequent insertion of the bronchoscope down the endobronchial lumen may be used to confirm that endobronchial placement is not too distal and that, with right DLTs, the right upper-lobe bronchus is over the corresponding fenestration. Although not essential for DLT placement, bronchoscopy reliably confirms accurate placement and should be used routinely. It has an advantage with anatomic variations and in detecting partial airway occlusions.17
Leak test. While ventilating one lung, a connection to the second airway can be placed under water. Any bubbling indicates air leak from the ventilated lung to the opposite side. Such leaks may not be detected by auscultation or bronchoscopy and may be important, particularly when one of the goals is to protect one lung against fluid (whole-lung lavage, blood, or purulent secretions) from the other lung. Bubbling may indicate the need for tube repositioning or higher bronchial-cuff inflation.
Chest radiography may be used to visualize DLT position, but is insufficient to verify critical tube placement or functional isolation.
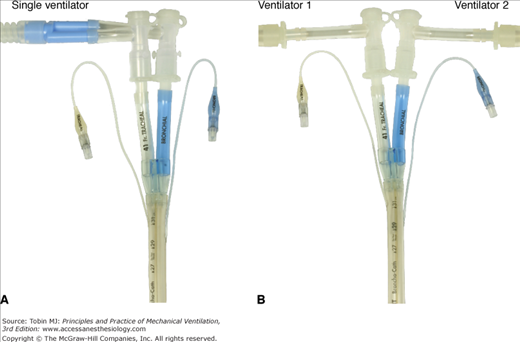
DLTs may be connected to a single ventilator (e.g., for hemoptysis or at the beginning and end of whole-lung lavage; Fig. 25-3A) or connected to two separate ventilators (see Fig. 25-3B).
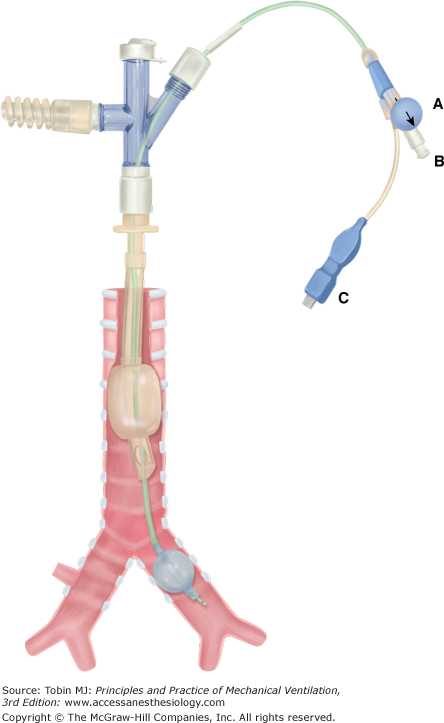
Bronchial blocking techniques and selective endobronchial intubation are alternatives to a DLT. The right or left main bronchus may be blocked by placement of a balloon-tipped catheter into that bronchus. This allows OLV and is suitable for thoracic surgery, bleeding, or fistula control. The catheter (Arndt endobronchial blocker, Magill blocker, Cohen Flexitip Endobronchial Blocker, Fogarty or Foley catheter)24–28 may be placed outside or within a standard cuffed endotracheal tube (ETT) lumen or passed down a specially designed second small lumen in the ETT (Univent tube).29–31 A Univent tube (Fig. 25-4) has a coudé-tipped bronchial blocker that allows blind guidance of the blocker into the desired bronchus with auscultatory confirmation of correct positioning. Bronchoscopic confirmation of best position within the airway, however, is still recommended. In addition, the Univent’s axial-blocker shaft has a lumen that allows irrigation, suction, O2 insufflation, CPAP, and high-frequency ventilation.29 The Arndt endobronchial blocker24–26 (Fig. 25-5) requires bronchoscopic guidance for placement. The kit comes with an ETT adaptor that allows access for mechanical ventilation, the blocker, and the bronchoscope through separate ports (Fig. 25-5). The bronchoscope is passed into the airway that requires blocking, and the blocker is then guided into that airway via a snare over the bronchoscope (Fig. 25-5A). The bronchoscope then is withdrawn, the balloon is inflated, and its position is confirmed by bronchoscopy before withdrawal (Fig. 25-5B). This has the advantage of being performed via the ETT in situ, thereby avoiding reintubation, provided that the ETT is sufficiently large to admit both the blocker and the available bronchoscope. The Cohen Flexitip Endobronchial Blocker28 has a flexible tip that can be guided under bronchoscopy but independently of the bronchoscope (Fig. 25-6).
A variety of techniques have been reported.
Synchronized independent lung ventilation (SILV) consists of synchronous initiation of inspiration into each lung. Each lung must necessarily have the same respiratory rate, but may have different tidal volume (VT), PEEP, and inspiratory flow. SILV may be achieved by a variety of techniques.
Two ventilators of the same type linked to cycle synchronously. This may be achieved by electronically “slaving” the rate control of a second ventilator to a primary (“master”) ventilator,18,32–40 by electronically synchronizing both to an external control device,41,42 or by simultaneously resetting the respiratory cycle on paired ventilators and relying on accurate internal timing to maintain synchronization.43,44 These forms of SILV allow difference in all variables apart from rate—different VT, PEEP, and inspiratory flow, and hence different inspiratory-to-expiratory time (TI/TE) combinations. SILV with two ventilators synchronized 180 degrees out of phase45,46 has been successful in animals, but appears to have no advantages over other forms of ILV and has not been reported in humans.
A single ventilator linked to a twin circuit with variable resistances in each inspiratory line47–49 can create different flows to each lung. The VT received by each lung will then be determined by both the resistance in the circuit and the impedance in the lung and must be independently measured in each circuit and resistance adjusted accordingly. An alternative to this is flow controllers in both inspiratory lines50,51 resulting in a fixed VT to each lung determined by the set flow and inspiratory time and independent of lung impedance. Separate PEEP in each circuit was achieved by expiratory flow controllers. This method allows different VT and PEEP to each lung, but necessarily must have the same TI, TE, and rate.
A single ventilator linked to two circuits, each with a separate PEEP valve or other PEEP-generating device.52,53 This arrangement allows different PEEP to each lung. The division of VT between the lungs is not controlled independently, being determined by both the relative inherent impedance of each lung and the effect of PEEP on that impedance.
A single ventilator linked to two circuits with no attempt to influence the distribution of ventilation.13 This method generally is used during selective airway protection. The division of VT between the two lungs is determined solely by their relative impedance.
While many indications for ILV are suited to having the same ventilator rate to each lung, there is usually no particular benefit for exact coordination of two ventilators. The only exception may be the uncommon circumstance where patient-triggered ventilation is attempted.
Asynchronous independent lung ventilation (AILV) consists of completely independent ventilator techniques applied to each lung. It requires two separate ventilation devices. Options include:
Controlled (CMV) or intermittent mechanical ventilation to both lungs.9,54–62
CMV or intermittent mechanical ventilation to one lung and high-frequency jet ventilation (HFJV) to the other.57,63–66
CMV or intermittent mechanical ventilation to one lung and CPAP to the other.7,57,58,67
High-frequency oscillatory ventilation to both lungs.68
AILV permits different rate, VT, inspiratory flow, and PEEP to each lung. Lack of synchronization between the two lungs offers the greatest flexibility and appears to hold no disadvantage and a number of advantages compared with SILV.55,61
With OLV, one lung is ventilated mechanically while the other is either occluded or open to atmosphere with an option of spontaneous ventilation. OLV is used mainly in thoracic surgery to keep the lung collapsed and immobile. It also may be used in bronchopleural fistula (BPF) to prevent air leak or for selective airway protection if secretions from the affected lung prevent any useful ventilation (e.g., massive hemoptysis). Options include:
DLT intubation11,20,21,69–71 or in infants, two separate uncuffed tracheal tubes of different lengths attached longitudinally (Marraro DLT)69 with CMV applied to only one lumen.
Endotracheal intubation with a bronchial blocker inserted into one of the major bronchi. The occlusive balloon excludes that lung from mechanical ventilation and the blocker lumen allows deflation (and inflation) of that lung.1,21,24–26,30
Endobronchial intubation with a single lumen tube (e.g., Mackintosh-Leatherdale left endobronchial tube and Gordon Green right endobronchial tube).21
Spontaneous ventilation with a DLT and differential CPAP applied to each lung was first reported by Venus et al.72 It has been used in spontaneously breathing patients with unilateral pulmonary contusion and atelectasis with good results.72,73 It is not recommended because of increased work of breathing through a long narrow tube.
Applications of Independent Lung Ventilation
|
A number of thoracic surgical procedures require OLV, usually in the lateral position. Table 25-1 lists the common thoracic surgical indications for OLV.19,21,69 Although OLV is required during surgery, ILV may be required before, and sometimes after, the surgical procedure.69,74
OLV may be achieved with a DLT, bronchial blocker, or endobronchial tube. A DLT has the advantage of enabling ILV, which may be important in alleviating hypoxia, but has the disadvantage of high-resistance airways with more difficult suctioning. An ETT with a bronchial blocker has the advantage of a wide-bore, low-resistance endotracheal lumen with better bronchoscopic and suction access, through which either OLV (bronchial blocker inflated) or double-lung ventilation (bronchial blocker deflated) can be delivered.30 The narrow lumen in some bronchial blockers can enable lung inflation, deflation, CPAP, or HFJV, but does not allow conventional ILV. OLV may be delivered to either lung, but repositioning of the bronchial blocker is required.30 When the blocker is an integral part of the ETT (Univent tube, see Fig. 25-4),29–31 it is easier to insert and it maintains a more stable position compared with an intraluminal or extraluminal blocker that is more subject to movement and dislodgment, especially during suctioning, bronchoscopy, or patient movement.30 With any bronchial blocker, bronchoscopy is easier and required less frequently.19,30 A disadvantage is that the inflated blocking balloon near the site of intended bronchial surgery (e.g., pneumonectomy, single lung transplantation) may hamper that procedure, and a DLT placed in the opposite lung may be technically easier.
Irrespective of patient position, gravity-induced differences in ventilation and perfusion occur vertically within each lung: between the bases and apices in the erect position, and between the dependent and nondependent lung regions in the supine position.
When a patient is in the erect or supine position, gravitational differences in ventilation and perfusion occur vertically within each lung. In the lateral decubitus position, these gravitational differences occur between the two lungs.71,75 Up to two-thirds of perfusion shifts to the dependent lung.75–77 During spontaneous ventilation, a smaller increase in ventilation also occurs in the dependent lung in the lateral position, thereby reducing ventilation–perfusion mismatch.71,75 When the patient is anesthetized and mechanically ventilated in the lateral decubitus position, however, reduced diaphragmatic activity allows the weight of abdominal contents to retard expansion of the dependent lung,71,75 and most ventilation is diverted to the nondependent lung. In this situation, the nondependent lung may receive up to two-thirds of ventilation.36,40,76 Opening the nondependent thorax further reduces ventilation to the dependent lung by facilitating expansion of the nondependent lung.77,78 The net effect of these changes is overperfusion relative to ventilation (or shunting) in the dependent lung and overventilation relative to perfusion (or dead space ventilation) in the nondependent lung with adverse effects on gas exchange.
Left thoracotomy achieves a higher partial pressure of arterial oxygen (PaO2) during OLV than does right thoracotomy because the left lung normally receives 10% less cardiac output than the right lung.70 The presence of chronic airway obstruction (CAO) is associated with better PaO2 during OLV possibly because of dynamic hyperinflation in the dependent lung.70 PaO2 during double-lung ventilation is also predictive of during PaO2 OLV.
Some forms of ILV cannot be applied when the nondependent lung is immobile, deflated, or removed, yet ILV has been shown to improve many of these abnormalities. Application of PEEP to both lungs improves dependent-lung ventilation and oxygenation.76 SILV (see “Techniques for Independent Lung Ventilation”) with delivery of equal VT to both lungs,39 or PEEP to the dependent lung,37,38,40,79,80 or both,38 improves oxygenation when compared with double-lung ventilation or generalized PEEP.
When OLV is undertaken and the nondependent lung is excluded from ventilation, the reduction in ventilation to the dependent lung is eliminated, but all perfusion through the nondependent lung constitutes a shunt. In this situation, the amount of perfusion of the nondependent lung is a major determinant of hypoxia. Hypoxic vasoconstriction (not opposed by anesthetic agents), lung collapse, and surgical occlusion of blood flow (pneumonectomy) to the nondependent lung all have the potential to reduce shunt and improve PaO2 in OLV.
PEEP in the dependent lung may be beneficial by increasing functional residual capacity and improving distribution of ventilation or be detrimental by increasing alveolar pressure and diverting blood flow to the nonventilated lung.71,81–86 Consequently, PEEP to the dependent lung during OLV may improve PaO2,81,86 decrease PaO2,81,82,86 or cause no change.84–86 During OLV, Cohen et al79 compared PEEP (10 cm H2O) to the dependent lung, CPAP (10 cm H2O) to the nondependent lung, and both. All three maneuvers increased compared with OLV alone. PEEP caused the smallest (nonsignificant) increase in PaO2. CPAP and PEEP + CPAP caused larger (significant) increases in PaO2, whereas PEEP + CPAP reduced cardiac output and oxygen (O2) delivery significantly, which CPAP alone did not. Cohen et al28 concluded that CPAP alone (to the nondependent lung) had the most beneficial effect by diverting blood flow to the dependent lung without reducing cardiac output. More recently, PEEP has been shown to benefit oxygenation during OLV to the dependent lung during thoracotomy.87
Because of these variable effects, it has been recommended that CPAP (5 to 10 cm H2O) be applied to the nondependent lung, combined with no, low, or high PEEP (5 to 15 cm H2O) to the dependent lung, depending on patient response.
Although function of the two lungs commonly differs after thoracotomy, conventional mechanical ventilation or spontaneous ventilation are usually resumed after surgery. ILV is required occasionally in the postoperative period for marked asymmetry of lung function with hypoxia,7,8,55 BPF,47,58,63,66,88 or following esophagectomy.74 Pawar et al69 reported successful use of the Marraro pediatric double-lumen tube89 (two separate uncuffed tracheal tubes of different lengths attached longitudinally) in seventeen children, ages 1 day to 3 years (2.7 to 12 kg) who required OLV during cardiothoracic surgery. Of these, six children required ILV up to 48 hours postoperatively. All children survived. Ito et al90 reported ILV with a separate bronchial cannula in each main bronchus, combined with bilateral HFJV, the repair of tracheal stenosis in a 2-year-old infant.
Pulmonary alveolar proteinosis is the most common indication for whole-lung lavage. This condition was first described in 1958,91 and soon after, bronchopulmonary lavage became established as the main treatment.92–100
Pulmonary alveolar proteinosis is most often acquired (90%) but may be congenital or secondary to conditions such as acute silicosis and other inhalational syndromes, immunodeficiency disorders, malignancies, hematopoietic disorders, and lysinuric protein intolerance.101 It has also been reported following prolonged cotton dust exposure.102 Recent studies suggest that acquired pulmonary alveolar proteinosis may be caused by deficiency of granulocyte-macrophage colony-stimulating factor,101,103 and administration of this factor is of value in approximately 50% of patients. Despite these advances, lavage remains the most effective therapy.101,103
The clinical course is variable: increasing dyspnea progressing to respiratory insufficiency, static disease with minimal symptoms, asymptomatic disease, and spontaneous resolution in some patients.101,104 Because the course is variable, the decision to undertake bronchopulmonary lavage is based on disease progression and symptoms.
Whole-lung lavage has been used for asthma,105,106 chronic bronchitis, and cystic fibrosis,11,98,105–111 but with doubtful benefit; it is no longer recommended. Radioactive dust inhalation is another indication.112
To minimize hypoxia during the procedure, the worst lung should be lavaged first. The worst lung can be identified by chest radiograph, ventilation–perfusion scan, or oxygenation3,113,114 during OLV to each lung before the procedure.
The procedure usually is performed with anesthesia, neuromuscular paralysis,11,105,115 and a left DLT in the supine position. The supine position is chosen to balance the risk between hypoxia and fluid spillage11 (see “Basic Principles of Independent Lung Ventilation”).
Complete lung isolation to prevent spillage of fluid from the lavaged lung into the ventilated lung is essential. Correct DLT position should be established using both bronchoscopy and leak testing (as above) up to plateau pressures of 40 to 50 cm H2O.11,19 Both lungs should be preoxygenated with 100% O2 to maximize gas exchange and eliminate nitrogen, which may prevent full access of lavage fluid to the lung to be lavaged.
Isotonic saline, warmed to body temperature, then should be infused through wide-bore tubing into the lung to be lavaged from a gravitational height 30 cm above the midaxillary line.11 This infusion pressure of 30 cm H2O usually results in infusion volumes of 500 to 1000 mL depending on the compliance of the lung. Efflux of fluid may be commenced as soon as fluid influx is complete; the drainage tube is placed below the patient, assisted by head-down posturing, and percussion and vibration of the hemithorax.11,115 Fluid flow may be achieved using separate clamped inlet and outlet lines or a single line that is elevated for fluid influx and lowered for fluid efflux. Total fluid exchange may range from 10 to 50 L and should be continued until efflux fluid is relatively clear.
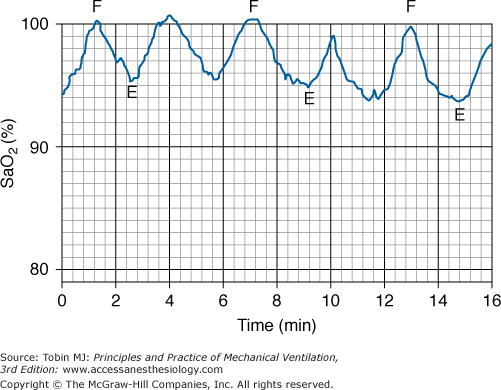
When fluid influx into the lavaged lung is complete, the alveolar pressure usually exceeds the pulmonary capillary pressure, minimizing shunt through this lung and maximizing arterial oxygenation at this stage of the procedure.105,115–118 When the lavaged lung is emptied of fluid, blood flow returns, and significant hypoxemia can occur (Fig. 25-7).11,105,115–117
Figure 25-7
Variations in arterial oxygen saturation (SaO2) during the influx (F) and efflux (E) phases of whole-lung lavage. (Modified, with permission, from Claypool et al.115)
On conclusion of the lavage, double-lung ventilation should be recommenced with the DLT in situ using a single ventilator and a bifurcated circuit. If oxygenation is satisfactory, the patient may be weaned and extubated or reintubated with a regular ETT and later weaned. If oxygenation is unsatisfactory, ILV may need to be recommenced. Up to 1000 mL of saline may be retained in the lavaged lung;115 although this is absorbed rapidly, lung function may not improve for hours or days. The procedure is repeated on the second lung after an interval of 1 to 3 days.
Leakage of fluid into the ventilated lung may be recognized by desaturation, crepitations, and rhonchi in the ventilated lung; fluid in the tubing to the ventilated lung; or air bubbles in the fluid from the lavaged lung.11 If this occurs, lavage should be ceased, and the patient should be placed in the lateral decubitus position with the lavaged lung dependent and the head down to facilitate drainage from both lungs. Active suctioning of both lungs should be undertaken. If the leak is only minor and adequate oxygenation is restored, the correct DLT position may be reestablished, lung isolation rechecked, and the procedure continued. With a major leak and failure to restore adequate oxygenation once fluid removal is complete, double-lung ventilation with PEEP should be resumed and further lavage delayed until hypoxia has improved.
Hypoxemia during the procedure is common. Some patients with severe lung disease are too hypoxemic before the procedure to tolerate OLV. Several options exist for such patients. Extracorporeal membrane oxygenation (ECMO) may be instituted before lavaging the first lung.11,119–123 ECMO may or may not be required for lavage of the second lung, depending on the effect of the first lung lavage on subsequent oxygenation. Cohen et al121 reported successful lavage of both lungs with ECMO during a single session, avoiding the need for a second procedure. Because of the complexity and limited availability of ECMO, an alternative is to perform limited bronchoalveolar lavage using a fiberoptic bronchoscope,124,125 with or without an inflatable cuff on the bronchoscope, under local anesthesia. This may be undertaken in a spontaneously breathing patient or during CMV through a single-lumen ETT. Limiting lavage to a lobe or several subsegments necessitates multiple procedures but minimizes hypoxemia. Nadeau et al126 reported that the combination of inhaled nitric oxide and inflation of a balloon in the right pulmonary artery during right whole-lung lavage improved oxygenation sufficiently to avoid ECMO.
Randomized studies of whole-lung lavage for pulmonary alveolar proteinosis have not been undertaken. Seymour and Presneill101 analyzed survival of 231 patients in multiple reports; 5-year actuarial survival from diagnosis was 94% ± 2% in patients who underwent whole-lung lavage (n = 146) compared with 85% ± 5% for patients who did not (n = 85). In fifty-five patients in whom duration of benefit was reported,101 median duration of benefit from lavage was 15 months; fewer than 20% of patients followed beyond 3 years remained symptom-free. In a series of twenty-one patients followed prospectively after whole-lung lavage, Beccaria et al127 found that more than 70% remained free from recurrent pulmonary alveolar proteinosis at 7 years. Although whole-lung lavage is labor-intensive, these data suggest that it is worthwhile.
Massive hemoptysis carries a high mortality128–130 and requires prompt intervention. Common causes include tuberculosis, bronchiectasis, lung abscess, mycetoma, pulmonary carcinoma, cystic fibrosis, arteriovenous malformations, and trauma.104,128,131–133 The source of bleeding is the bronchial arterial system in 90% of patients.134 Iatrogenic causes are uncommon but include pulmonary artery rupture by a pulmonary artery catheter128,135,136 and transbronchial lung biopsy. Death results from acute asphyxia and is related to the rate and volume of blood loss and the underlying condition.13,128,129,137,138 Factors increasing mortality include preexisting pulmonary insufficiency, obtundation from any cause, poor cough, and coagulopathy.129,137,139,140
Management consists of general measures, diagnosing the site of bleeding, isolating the bleeding lung, and controlling the bleeding. The patient should be given 100% O2, placed in the head-down lateral-decubitus position, bleeding side down, clotting studies performed, blood typed, wide-bore intravenous access established, and cough suppressed with an opiate, and resuscitation and suction equipment should be in close proximity. Chest radiography and computed tomography scanning should be done when appropriate.
A number of alternatives exist for the localization, isolation, and control of the bleeding:
Fiberoptic bronchoscopy and placement of an endobronchial blocker (Fogarty catheter or Arndt endobronchial blocker) in the bleeding lung or segment should be done.25,26,141–143 This can be performed in a patient who is not intubated but is easier in one who is. Fiberoptic bronchoscopy is limited by the narrow suction channel and rapid visual loss secondary to occlusion of the lens by blood.140 Fiberoptic bronchoscopy, however, can allow accurate localization of bleeding,104,128,140,144,145 catheter placement in a bronchial segment,128,141,146 and lavage with iced isotonic saline or epinephrine.128,146
Rigid bronchoscopy has advantages over fiberoptic bronchoscopy when blood loss is massive because of better suction, visual access, and airway control.137,139,140,144,147,148 It allows easier placement of endobronchial blockers and iced saline or epinephrine lavage. It also allows diathermy, laser therapy, and cryotherapy,130 as well as placement of endobronchial tampons soaked in vasoconstrictor drugs.137 As use of embolization increases, rigid bronchoscopy is required less, although it still has a place.144,147
Endotracheal intubation may be required where bleeding is sufficient to compromise oxygenation, particularly if mentation is depressed or cough is inadequate. If bleeding is so rapid that acute asphyxia arrest is imminent despite intubation,128 the ETT can be advanced beyond the carina (usually into the right main bronchus) and OLV commenced.128,149 If blood does not flow out of the ETT (implying blood loss from the contralateral side), the cuff is inflated and the ETT left in situ. If bleeding continues through the ETT, a Fogarty or Foley catheter is passed, the main bronchus is occluded, and the ETT is withdrawn to the trachea to ventilate the contralateral side.
Selective intubation is performed with a small (6 to 7 mm) ETT, with or without fiberoptic bronchoscope guidance, or a selective left or right endobronchial tube.128,137 Selective intubation must be preceded by accurate bronchoscopic localization of bleeding because it excludes one lung from ventilation. Selective intubation has the advantage of reliable protection of the nonbleeding lung137,140 but the disadvantage of permitting OLV only and excluding the bleeding lung from endobronchial procedures and suctioning. Bleeding then must be controlled by tamponade, bronchial angiography, and embolization or surgery.
DLT insertion is an alternative that isolates the lungs but preserves access to both. In the past, DLTs were not recommended as an early alternative128,137 to control bleeding. Problems included difficulties with insertion under adverse circumstances, requirement for an experienced operator, difficulties with suction and bronchoscopy access, and easy blockage of DLT lumen with clot. More recently, use of PVC DLTs has been successful.13,131,150 DLT enables lateralizing of the bleeding, protects the nonbleeding lung, allows ILV, and allows therapeutic procedures to address the bleeding lung without compromise of the healthy lung.13,150 A left DLT is the tube of choice.13,131,137,150 Most commonly, a single ventilator with a Y connection to the DLT lumens is used13 with distribution of ventilation according to the relative impedance of the two lungs (see “Lung Separation” above). If there is risk of blood overflowing to the nonbleeding lung, or if differential ventilation is required, a second ventilator with AILV should be used. OLV must be present during bronchial blockade or endobronchial intubation and may be required during iced saline lavage or massive blood loss.
Embolization. Because 90% of major hemoptyses arise from bronchial arteries,134 once bleeding has been localized, bronchial artery embolization has a high success rate.104,134,145,151–153 Once the bleeding lung or segment has been isolated, the bleeding must be controlled.
Emergency surgery. Urgent surgical resection is undertaken if bleeding overwhelms airway control or fails to respond to other measures. Resection is associated with a low mortality in many series.104,129,140,144,152,154 It should not be unduly delayed if bleeding is not readily controlled.
The choice of these alternatives depends on the intubation status of the patient, the rate of bleeding, and whether the site of bleeding is known (Table 25-3). The choice is also affected by the availability of the technique and the skills of the personnel involved. In an unintubated patient who remains stable despite moderate hemoptysis, bleeding may settle with correction of clotting abnormalities and general measures (above) or angiographic embolization (Table 25-3). An obtunded patient with a decompensating respiratory or circulatory state requires intubation combined with a plan to confine the bleeding to one lung (Table 25-3). This can include a primary choice of specialized tubes, such as the Univent or a DLT. A patient who is already intubated will usually have control attempted primarily by a blocker or manipulation of the ETT in situ (Table 25-3).
| Intubation | Severity | Side of Bleed | Management Options | |
|---|---|---|---|---|
| A | Not required | Moderate | Conservative only | |
| Angiogram and embolization | ||||
| Elective intubation and blocker | ||||
| B | Required | Moderate | ETT + blockera ± bronchoscopic guidance | |
| or massive | Known | Univent tube ± bronchoscopic guidance or DLT | ||
| Massive | Known left | ETT advanced into R main bronchus | ||
| Massive | Unknown | DLT or ETT to right main ± blockera and withdraw ETTb | ||
| C | Already present | Moderate | Blockera ± bronchoscopic guidance | |
| Massive | ETT to right main ± blocker and withdraw ETTb |
In reported series of patients with major hemoptysis, localization of bleeding is usually achieved by history, fiberoptic bronchoscopy, or radiology (chest radiograph, computed tomography scan, or angiography). Bleeding is controlled by conservative measures, embolization, or surgery.104,145,152 The relative requirement for these three treatments varies widely,104,145,152 depending on cause, amount of bleeding, and local expertise. Supportive care, correction of coagulopathy, and time as the only measure ranged from 13% to 87% of patients with major hemoptysis. Embolization was used in 7% to 51% with success rates of more than 80%. Surgery was required in 6% to 50%.
Spread of purulent secretions from a lung abscess, empyema, bronchiectasis, cavitating tuberculosis, or cavitating malignant disease to the dependent normal lung during chest surgery is associated with considerable risk.1,2,5,6,155 A DLT not only allowed thoracic surgery but provided an important protective role for the dependent normal lung1,66,155 and allowed perioperative ILV if required.1,66,155
Although these conditions have become less common, the requirement for ILV for lung protection, during or outside thoracic surgery, occurs occasionally.156 Essential during thoracic surgery, it is problematic outside that setting because viscous or tenacious secretions drain poorly through the narrower lumen of a DLT. Appropriate antibiotic therapy, postural drainage, and a standard ETT may be preferable.
Mainstem bronchi and lower trachea (near the carina) can be ruptured by severe blunt chest trauma, resulting in tension pneumothoraces, massive air leak, and the need for urgent surgical repair. Selective airway intubation usually is required for the repair and ILV has been used postoperatively to protect the anastomoses.157–160 Pizov et al160 used one-lung high-frequency ventilation in the management of traumatic tear of the bronchus in a child. After a right mainstem bronchial rupture, Moerer et al157 used a left DLT with bilevel ventilation to the left lung and CPAP alone to the right lung for 48 hours before switching to CMV. In three patients with lower tracheal rupture (near the carina), Wichert158 reported that standard DLTs position the cuff too close to the site of carinal injury and used bronchoscopy-guided selective endobronchial intubation with two tubes to undertake the repairs. After repair, the tubes were reintroduced via tracheostomy, and ILV was performed for 9 to 14 days to allow recovery from respiratory and other complications.