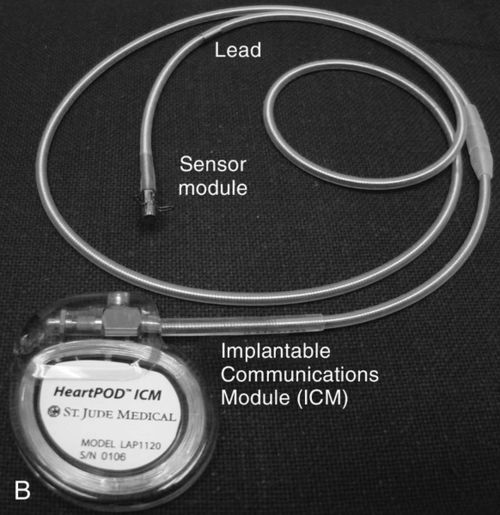

The left atrial pressure sensor and the HeartPOD system were being studied in a randomized controlled study titled the Left Atrial Pressure Monitoring to Optimize Heart Failure Therapy (LAPTOP-HF) trial, however, patient enrollment was stopped. The purpose of LAPTOP-HF was to evaluate the safety and clinical effectiveness of using left atrial pressure measurements and a management system that was physician directed and patient managed. The system guided adjustments in heart failure medications (similar to insulin therapy being guided by blood sugar levels). Clinical effectiveness may be measured by episodes of worsening heart failure and hospitalizations, and patients will be compared on the basis of being managed with the left atrial pressure system or usual care.37
CardioMEMS HF System
The third device is the CardioMEMS HF System (developed by CardioMEMS, Atlanta, GA and sold to St. Jude Medical, St. Paul, MN, in 2014), a fully implantable, wireless pulmonary artery device that monitors pulmonary artery systolic, mean, and diastolic pressures.38 This device was approved for clinical use by the FDA in May 2014. CardioMEMS uses a lead-less sensor that is placed in a distal branch of the pulmonary artery (Figure 13-3), home electronics that allow for daily pressure readings to be transmitted to health care providers, and a trend report of pressure information and individual waveforms. The sensor itself is a very small capsule covered by silicone (15 millimeters [mm] in length, 3.4 mm in width, and 2 mm in thickness) that contains a coil and pressure-sensitive capacitor. 38 Two nitrol loops, one on each end of the sensor, maintain the sensor in place in the pulmonary artery. No batteries need to be placed in the sensor, as an external antenna from the home electronics is held against the patient’s body to create electromagnetic coupling and power the device. The antenna continuously measures resonate frequencies, which are converted into pressure waveforms, based on an electrical circuit that is created by the coil and capacitor.38
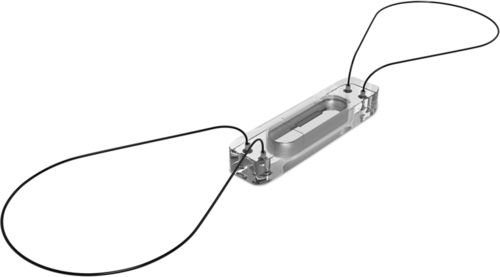
In 2011, the outcomes of the CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Heart Failure Patients (CHAMPION) trial were reported.39 The trial involved implantation of a CardioMEMS sensor in 550 patients, a one-night hospital stay, and training of patients to use the home electronics. All patients were told to take daily pressure readings, which were uploaded to a secure patient database. Data from the sensor was only available to patients randomized to the treatment group. Company-sponsored nurse communication was also available to the treatment group. When the pulmonary artery pressures exceeded the protocol-defined treatment goals and when signs and symptoms of worsening heart failure were identified on the basis of standard of care practices, physicians were expected to use neurohormonal, diuretic, and vasodilator heart failure therapies to reduce pressures and alleviate symptoms.39 The usual care group received alterations in heart failure therapies based on standards of care. At 6 months and during the entire randomization period, patients in the treatment group had significant reductions in heart failure–related hospitalizations, and compared with the usual care group, the treatment group had a significant reduction in mean pulmonary artery pressure and more days alive outside of the hospital.39
Because of concerns regarding the effects of nurse communication to enhance protocol adherence in the treatment group of the CHAMPION trial, a 13-month open access assessment period (part 2) followed the 17.6-month randomized access data collection period (part 1, described above). In part 2, both the former usual care and the treatment groups received standard heart failure management and both had physician knowledge of pulmonary artery pressures. Moreover, neither group received nurse communications, thereby addressing issues that were raised during the initial FDA review.40 In part 2, 177 former treatment patients and 170 former usual care patients participated with 119 and 127, respectively, completing the open access assessment period. Data were analyzed for heart failure hospitalization rates.40
In part 2 analysis, physician access to pulmonary artery pressure measurements led to a significant reduction in the annualized heart failure hospitalization rate among patients in the open access control group compared with the rates from part 1, reflecting the benefit of physician access to hemodynamic data.40 Of the treatment group patients, no differences in annualized heart failure hospitalization rates existed between part 1 and part 2 groups, reflecting that the part 1 nurse communication component may not have had a large influence on physician treatment decisions.
Alternatively, physicians could have learned new management options to reduce pulmonary artery pressures during the randomized access period, then used these strategies during the open access period. Additionally, patient gains in part 1 associated with stabilization of hemodynamic status may have been maintained in part 2.
When the magnitude of change in annualized hazard ratios for heart failure hospitalization was assessed between part 1 and 2 of the control and treatment groups, a significant change (improvement) was seen in the control group (from 0.68 to 0.36) and no change in the treatment group (from 0.48 to 0.45), reflecting the benefit of access to pulmonary artery pressure monitoring in the former control group. Analysis also revealed that hemodynamic monitoring created early gains but that prolonged monitoring did not lead to a continued reduction in the annualized heart failure hospitalization hazard rate.40 Features of devices with hemodynamic monitoring features are provided in Table 13-1.
Table 13-1
Devices with Hemodynamic Monitoring Features
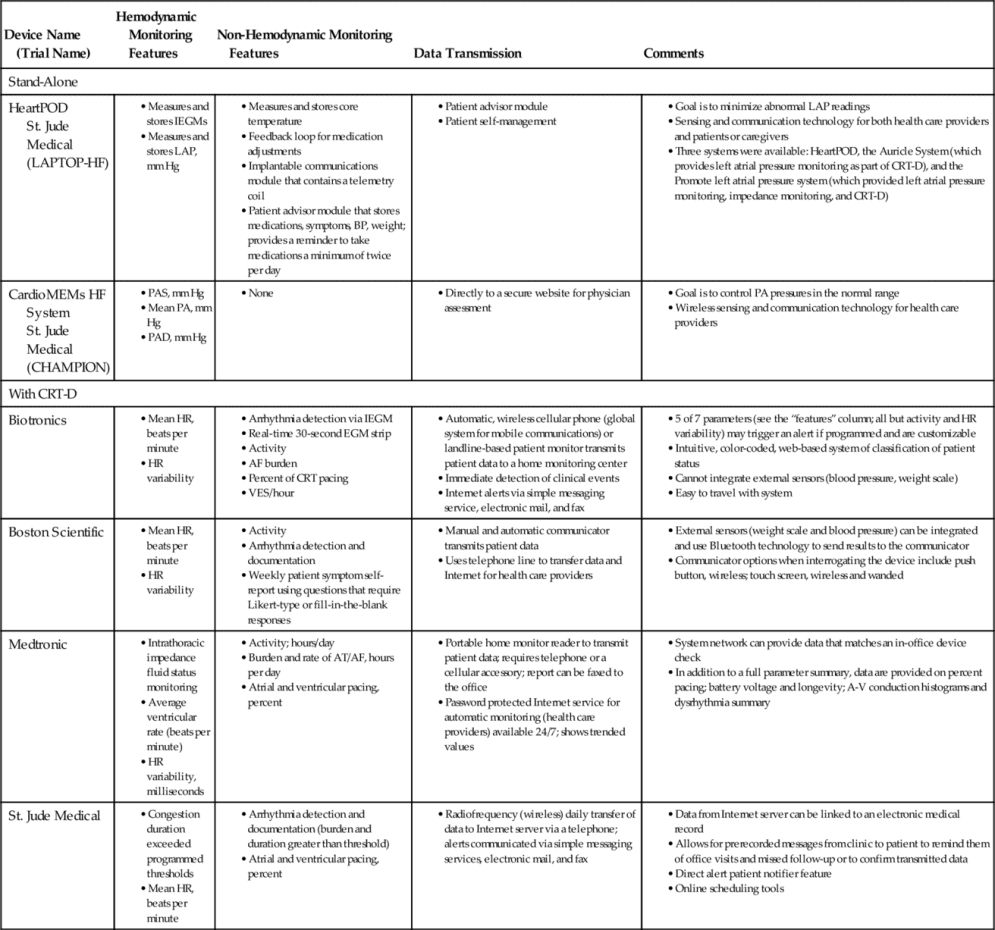
AF, Atrial fibrillation; AT, atrial tachycardia; BP, blood pressure; CRT-D, cardiac resynchronization therapy-defibrillator; D, diastole; HR, heart rate; IEGM, intracardiac electrogram; LAP, left atrial pressure; PA, pulmonary artery; S, systole; VES, ventricular episodes; VT/VF, ventricular tachycardia/ventricular fibrillation.
Monitoring Features of Implanted Cardiac Resynchronization Therapy-Defibrillator (CRT-D) Devices
In the early 2000s, two of the four major pacemaker device companies developed internal monitoring features for some models of cardiac resynchronization therapy (CRT) devices. Currently, internal monitoring features are available in all implantable CRT-defibrillator (CRT-D) devices, although the features offered by each company vary (see Table 13-1).
Most internal monitoring features of CRT-D devices are not hemodynamic monitoring specific; for example, recording patient activity level and percent of atrial and ventricular pacing. Other CRT-D devices record intrathoracic impedance, average ventricular day and night heart rate, and heart rate variability. The intrathoracic impedance feature provides a lung volume impedance measurement that reflects thoracic fluid, which is usually a surrogate for lung edema. High intrathoracic impedance and low fluid index values reflect a dry lung state. Thoracic fluid status can be monitored from the patient’s home as a stand-alone data point or with other CRT-D internal monitoring data and may be used by health care providers to facilitate outpatient management of heart failure.41,42 Data points or data trends could provide early warning that could facilitate treatment changes that prevent hospitalization.
The literature contains many case studies and research reports of health care providers’ use of internal monitoring data from internal cardiac devices. Clinicians described a decrease in intrathoracic impedance and a subsequent rise in fluid index that were associated with a decrease in patient activity, a rise in night-time heart rate, and a decrease in heart rate variability over time, which matched patients’ reports of worsening symptoms of heart failure. Further, trends were reversed once diuretics were administered.43,44 In 23 patients who had intrathoracic impedance validated by PAOP obtained by the echocardiography–Doppler method, the kappa-coefficient was 0.70 (standard error, 0.113), reflecting strong accuracy. Further, the impedance alert detected clinical heart failure deterioration with 92% sensitivity and 67% positive predictive value.45 Since the positive predictive value was only 67%, it must be remembered that not all changes in intrathoracic impedance were caused by lung edema.
Internal monitoring data was used in a multi-center, retrospective, cohort study, of impedance-monitoring capabilities via a CRT-D, where 326 patients were monitored for fluid index levels that rose above the threshold level (labeled as an “event”). Patients with more than three threshold crossings of fluid index values per year had a significantly higher likelihood of a heart failure hospitalization during the study period.46 Thus, serial decreases in intrathoracic impedance leading to high fluid index levels that cross established thresholds provide opportunities for health care providers to reassess current management strategies and ensure that guideline-directed medical therapies are optimized. Of note, a modified CRT-D algorithm was developed to detect a reference impedance and fluid index level. In a study of 81 subjects, the modified algorithm decreased unexplained fluid index threshold crossings.47 To date, the modified algorithm is not available clinically; a multi-center, randomized controlled study would be required prior to FDA approval.
Intrathoracic impedance values were also associated with hemodynamic and physiologic values. Researchers found strong correlations between stroke impedance and both stroke volume and pulse pressure.48 In another report of CRT internal monitoring features, persistent atrial fibrillation burden, for longer than 24 hours, worsened heart rate variability and activity level and increased night-time heart rate.49 Also, it took approximately 30 days after the atrial fibrillation episode ended before activity and heart rate variability levels returned to preatrial fibrillation values.49
In a study of patients who were monitored for 8.6 months, atrial tachycardia events preceded a fluid index event (reflecting a drop in intrathoracic impedance and presence of lung edema) in 43% of cases. Atrial tachycardia events followed a fluid index event in 29% of cases, and was simultaneous or intermediate in 22% of cases.50 Thus, patients with heart failure had increased susceptibility to temporary atrial tachycardia with worsening pulmonary congestion. In another study, researchers learned that ventricular tachydysrhythmic events occurred within 30 days of a fluid index threshold crossing event.51 This knowledge may lead to a better understanding of the interplay between atrial or ventricular tachyarrhythmias and neurohormonal and cardiac remodeling events in heart failure.
Using Implantable Hemodynamic Monitoring
Implantable hemodynamic monitoring may offer many benefits, but some potential issues have to be considered as well. In the case of both stand-alone devices and the internal monitoring features of CRT-D, no new knowledge is available that suggests that the device data can be used in isolation from other history and physical examination findings. Therefore, current benefits are relative in that device data must be coupled with other patient data before treatment plans are finalized, to ensure patient safety and minimization of adverse outcomes. In the following section, both benefits and potential issues are discussed.
Benefits
Thus far, pulmonary artery sensor technologies have been reasonably safe, accurate, and seemingly durable, although more research is warranted. An implantable hemodynamic monitor that wirelessly transmits pulmonary artery pressures to patients and health care providers allows for distant, ambulatory control of moderate to severe heart failure. In the case of internal monitoring features that are part of CRT-D, electrophysiology cardiologists have a long history of placing devices in patients. The technology is safe, and benefits of the core device (CRT, implantable cardioverter-defibrillator or CRT-D) are well known to outweigh risks. Both pulmonary artery catheter stand-alone devices and hemodynamic monitoring features that are part of CRT-D devices offer quick, timely and ongoing access to data that could effectively decrease heart failure events and acute decompensation requiring hospitalization. Freedom from heart failure events may improve heart failure–related pharmacologic profiles, quality of life, and clinical outcomes.
Potential benefits of implantable hemodynamic monitoring are associated with electronic data capture. If hemodynamic data can be downloaded to multiple health care providers, as needed, problem identification and optimal treatment planning might be maximized. Multi-disciplinary collaboration among health care providers might increase, creating uniformity in the plan of care revisions, and consistent messaging to patients and families.
Potential Issues
Potential issues with stand-alone hemodynamic monitoring involve the comfort and ease of health care providers in making decisions that impact patient outcomes. In the CHAMPION trial, the CardioMEMS system was not initially approved for use by the FDA because decision makers were unable to determine the effectiveness of the data in changing clinical practice.40 During the trial, company representatives advised physicians in dealing with abnormal hemodynamic values. Thus, the impressive reduction in hospitalization could have been caused by external support that would not be available once the device was approved for use in clinical practice. Considering that many patients with heart failure are managed by primary care providers, and not cardiac specialists, outcomes may be hard to replicate in clinical practice. When hemodynamic data are provided as stand-alone data, treatment decisions involving aggressive diuretic or vasodilator therapies could lead to hypotension, dizziness, or neurohormonal stimulation that worsens heart failure.52 If, indeed, the CHAMPION trial health care providers used other diagnostic and symptom measures such as B-type natriuretic peptide to make decisions, a need for transparency exists to learn how treatment decisions were really made so that the value of implantable hemodynamic monitoring can be accurately determined.
If a hemodynamic monitoring system has accurate, reliable, and safe medication management algorithms, both the potential issues of failing to act when pressures increase above present thresholds and overreacting to too-high pressures could be avoided, that is, assuming the algorithm was complete and health care providers followed the actions as determined. However, the problem with algorithm use is that many health care providers believe that the art of managing heart failure is just as important as the science of heart failure management. With an algorithm, specific circumstances that could have preceded the changes in hemodynamic pressures may not have been considered. Further, if medication management decisions are made without knowledge of special circumstances, changes in medication prescriptions may be incomplete or suboptimal.
Implantable hemodynamic monitoring is a new era in patient management that allows for data generation with simple patient actions or without patient interface. Since traditional history and physical examination data are not used to determine the cause of deterioration or improvement, patients may feel distanced from health care providers, especially when only red flags are acted upon. Patients may believe that heart failure self-care expectations are not necessary, or they may believe that they will be contacted only when a significant change in status requires attention, whether or not health care providers are really paying attention to incoming data. The patient may feel like a “patient,” rather than a person with heart failure, and resent expectations of daily activity associated with monitoring. Patients may have fears related to home electronics alarms. Thus, barriers to using telemonitoring may also be barriers to implantable hemodynamic monitoring.
Electronic data capture is a potential benefit of hemodynamic monitoring; however, it could also be a burden. Often, a large portion of downloaded data cannot be acted on. If episodic office-based follow-up is going to change to continuous or frequent device assessment, system changes will need to occur. Health care personnel will need to allocate time to be able to triage data and identify data abnormalities that require action. The downloaded implantable hemodynamic monitoring data will need to be reliable and trusted to result in economic and patient benefits. In one study of remote monitoring, some measurements did not correlate with others; for example, blood pressure did not correlate with weight, activity, or the difference between mean and resting heart rates.53 Measurements that are not associated with others and do not offer additional value need to be identified and isolated.
Management Considerations
Implantable hemodynamic monitoring requires specific nursing and physician considerations that relate to time, education, and systems to enhance teamwork and communication. Table 13-2 provides details of each consideration. To have a successful program, all areas must be addressed. Currently, most acute care and ambulatory nurses do not have the available time to download data from a company server, and to save data to an electronic medical record. Further, even though only a specific percentage of data might require a health care provider’s review and response, if the goal is to assess for trends of worsening hemodynamics before an impending emergency care visit or hospitalization, it might be necessary to build time into the work plan, especially if there is a need to download device data during an office visit or hospitalization.
Table 13-2
Management Considerations Specific to Implantable Hemodynamic Monitoring
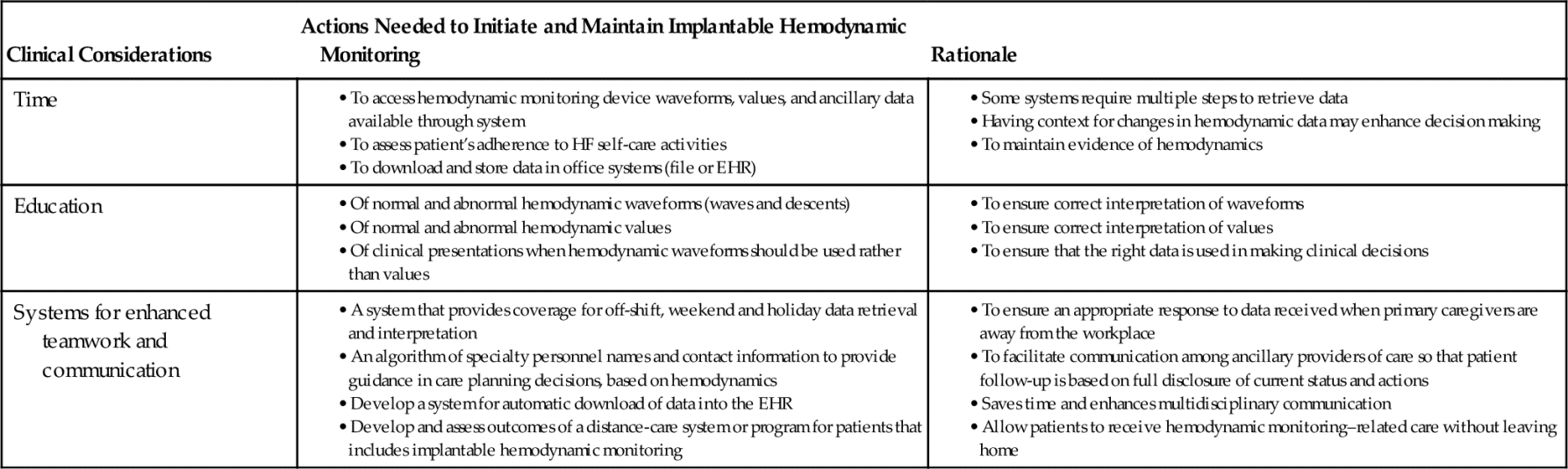
EHR, Electronic health record; HF, heart failure.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








