Home Mechanical Ventilation: Introduction
Over the last three decades, home mechanical ventilation (HMV) has become a widely accepted treatment option for patients with chronic hypercapnic respiratory failure that arises from different etiologies such as chronic obstructive pulmonary disease (COPD), restrictive thoracic disorders, neuromuscular disorders, and obesity hypoventilation syndrome.1,2 There is increasing evidence that HMV is capable of improving symptoms, health-related quality of life (HRQL), and long-term survival in most of these patients,1–3 although the impact of HMV on survival in patients with COPD is still a matter of debate.4,5 Thus, HMV should be considered in every patient presenting with symptomatic chronic hypercapnic respiratory failure.
In principle, HMV can be delivered by the application of long-term invasive mechanical ventilation, which requires the insertion of a tracheal tube following tracheostomy. Alternatively, noninvasive ventilation can be used to implement HMV via two possible routes. First, by application of negative pressure ventilation, which achieved international renown in the days when iron-lung ventilation was the preferred method for treating poliomyelitis; nowadays, this technique is seldom used.2 Second, noninvasive positive-pressure ventilation (NPPV) can be delivered by connecting the natural airways of a patient and the artificial airways of the ventilator system by the use of face masks, which cover either the nose alone (nasal masks) or both the mouth and nose (oronasal masks).6 Mouthpiece ventilation is an additional option, particularly for neuromuscular patients.7
According to a large European epidemiologic study covering more than 21,000 HMV patients from sixteen different countries (Eurovent survey), the overall prevalence of HMV reportedly was 6.6 per 100,000 inhabitants.8 Substantial variation, however, has been identified among countries in terms of (a) prevalence, (b) the relative proportions of specific patient groups receiving HMV, and (c) HMV techniques. Nevertheless, the number of HMV patients is steadily increasing,9 and the Eurovent survey only refers to the time span of 2001 to 2002.8 Moreover, the survey was confined to selected HMV centers that were invited to participate, whereas HMV is increasingly being implemented by many hospitals that are not officially known as HMV centers. Thus, the prevalence of HMV varies substantially between different countries and is at present much higher, at least in some Western countries, than what was estimated by the Eurovent study. Of similar importance is the fact that the pattern of different conditions underlying chronic respiratory failure is changing over time, with patients suffering from COPD and obesity hypoventilation syndrome experiencing the largest increase in prevalence.9
The Eurovent study also identified 13% of the survey population as recipients of invasive ventilation, with the highest percentage comprising patients with neuromuscular disorders (24%). Most patients received NPPV and only 0.005% received other forms unlike positive-pressure ventilation. Thus, NPPV has become the predominant means of delivering HMV.
Rationale
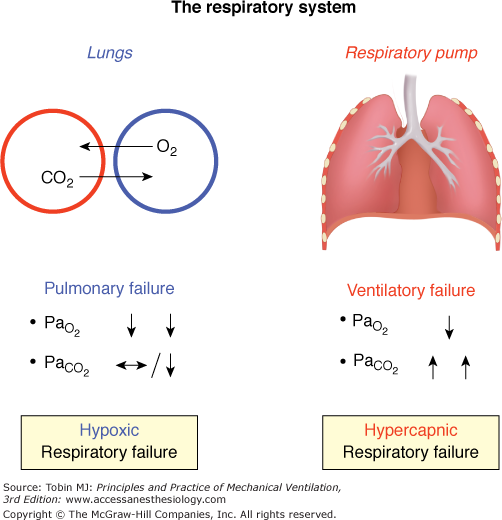
The respiratory system consists of two independent parts, each of which can be selectively impaired by different pathologies (Fig. 28-1):10 (a) the lungs, which are responsible for gas exchange, and (b) the respiratory pump, which regulates mechanical movements to ventilate the lungs.
Figure 28-1
The two components of the respiratory system. In the lungs, oxygen (O2) diffuses from the alveolus (blue) into the capillary (red), while carbon dioxide (CO2) diffuses from the capillary into the alveolus. The respiratory muscles (right panel: diaphragm, intercostal muscles and accessory muscles) are essential for sufficient respiratory pump function.  , partial pressure of oxygen;
, partial pressure of oxygen;  , partial pressure of carbon dioxide.
, partial pressure of carbon dioxide.
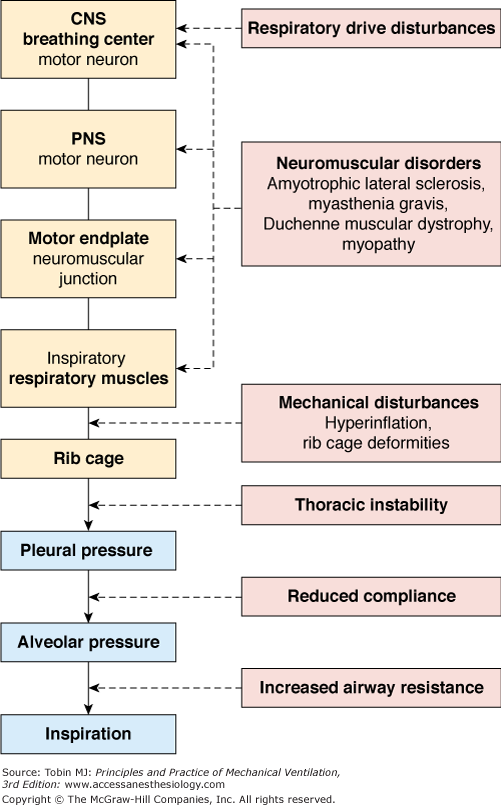
Basically, pulmonary insufficiency leads to hypoxemic respiratory failure, indicating impaired gas exchange; here, oxygen is primarily affected because of its poorer diffusion capacities compared to carbon dioxide. In contrast, ventilatory insufficiency primarily leads to hypercapnia, indicating reduced alveolar ventilation, although hypoxemia also occurs as a result of hypoventilation. This is most often the result of increased load on the respiratory muscles, decreased respiratory muscle capacity, or both, although it can also result from decreased respiratory drive.10,11
Treatment of chronic respiratory failure primarily depends on which part of the respiratory system is impaired. Chronic pulmonary failure is a well-justified basis for long-term oxygen treatment, with documented improvements in long-term survival in patients with COPD.12,13 In contrast, chronic failure of the respiratory pump coupled with reduced alveolar ventilation requires artificial augmentation of alveolar ventilation, which can only be achieved by long-term mechanical ventilation, that is, HMV. The indications for HMV are heterogeneous, in accordance with the complexity of the respiratory pump (Fig. 28-2).
Several trials have established that in most patient groups, NPPV used for HMV is capable of improving physiologic variables, the most important of these being blood gases.1–4,9,14–20 Conflicting results in patients with COPD have been published; the effect of NPPV on blood gases was negligible in some trials,5,21–23 but a positive effect was clearly documented in other trials.3,4,24–27 NPPV improves blood gases not only during the time of application, but also during subsequent spontaneous breathing. This benefit has been attributed to an improved breathing pattern with increased tidal volume at an unchanged respiratory rate.18
Although the mechanisms by which intermittent NPPV improves subsequent spontaneous breathing remain unclear, three theories have been advanced: (a) respiratory muscle resting; (b) resetting of CO2 sensitivity in the central breathing center; and (c) changes in pulmonary mechanics.1,2,20,28,29 The mechanisms are considered complementary rather than contradictory.1,2 It is, however, still unclear if these mechanisms are unique to all conditions leading to chronic ventilatory failure or are disease related. More recent research suggests that the principal mechanism in patients with restrictive thoracic disorders is an increased ventilatory response to CO2.20
Regarding the assessment of respiratory muscle strength, there is a lack of reliable methodology available. Volitional tests such as the widely used maximal inspiratory pressure method are highly dependent on the patient making a truly maximal effort.30 In addition, normal values rely greatly on the study cohort and on several methodological aspects; this has resulted in contradictory regression equations for calculating normal values for maximal inspiratory pressure.31 Thus, this technique is not suitable for physiologic studies. In studies using nonvolitional gold-standard techniques, however, no changes in diaphragmatic muscle strength—as assessed by transdiaphragmatic twitch pressures following magnetic phrenic nerve stimulation—could be observed following initiation of HMV in restrictive thoracic disorders20 and in COPD.32
Another theory for improved spontaneous breathing stems from reports of improved lung function in patients with COPD in whom a substantial reduction in partial pressure of arterial carbon dioxide (PaCO2) could be achieved.3,4,25,26 It has been speculated that the improvement results from a reduction in airway edema.25,33 Edema is a common finding in patients with COPD and might be related to cor pulmonale or possibly volume overload secondary to activation of sodium-retaining mechanisms. This, in turn, is thought to result from a reduction in effective circulating volume, secondary to a fall in total peripheral vascular resistance caused by hypercapnia-induced dilation of the precapillary sphincters.34 A decrease in hypercapnia by means of HMV would then reverse dilation of the precapillary sphincters and thereby impact positively on the edema, which could improve respiratory mechanics in patients with airway edema.
Selection of Patients
There are three mandatory conditions for commencing HMV:1,2,35 (a) presence of an underlying condition or disease that potentially causes impairment of the respiratory pump and ultimately chronic ventilatory failure; (b) typical symptoms accompanying chronic ventilatory failure, despite optimal treatment; and (c) evidence of a chronically decompensated respiratory pump with reduced alveolar ventilation.
Before the commencement of HMV, the cause of the chronic respiratory pump failure needs to be identified and all appropriate treatment strategies instituted. It is necessary to bear in mind that more than one condition can be responsible for the development of chronic ventilatory failure in some patients. Chronic ventilatory failure from the combination of COPD and obesity is especially common. All diseases affecting the respiratory pump may warrant HMV treatment, and the most frequently reported conditions are listed in Table 28-1.
|
No specific set of symptoms is reliably associated with chronic ventilatory failure because many occur in other conditions. In addition, symptoms (Table 28-2) depend on the underlying disease, its time course, comorbidities, and several other factors, such as medication and symptom perception. Some symptoms, however, are often falsely attributed to other conditions, leaving chronic ventilatory failure undiagnosed. Because one major goal of HMV is to alleviate symptoms, patients should be carefully screened for these symptoms both before and after the commencement of HMV (Table 28-2).
|
The most important indicator of reduced alveolar ventilation is an elevated level of partial pressure of carbon dioxide (PCO2), which can be detected by arterial or capillary blood gas analysis, end-tidal recording, or transcutaneous measurement (Table 28-3).
| Method | Advantages | Disadvantages |
|---|---|---|
| Arterial (PaCO2) |
|
|
| End-tidal (PETCO2) |
|
|
| Transcutaneous (PtcCO2) |
|
|
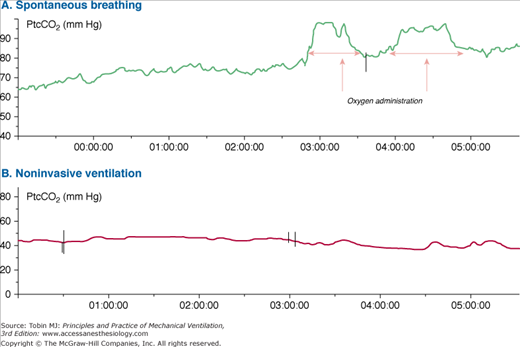
Although arterial measurement is regarded as the reference standard, transcutaneous measurements are increasingly recognized as being reliable. This has been attributed to technical refinements, which include the prevention and correction of measurement drifts.36,37 The advantage of transcutaneous measurements is that they enable noninvasive and continuous assessment. The technique is especially attractive during sleep, because sleep is not disturbed by an invasive procedure and it also captures any variations in alveolar ventilation (Fig. 28-3.)
Figure 28-3
Transcutaneous carbon dioxide tension (PtcCO2) monitoring in a 49-year-old patient with chronic ventilatory failure secondary to amyotrophic lateral sclerosis. A. PtcCO2 starts with values of 65 mm Hg during spontaneous breathing and progressively increases during sleep. Administration of oxygen produces an increase in PtcCO2, likely caused by reduced alveolar ventilation. B. Home mechanical ventilation by means of noninvasive ventilation produces normalization of PtcCO2.
Initially, hypercapnia typically occurs during the night, and the patient may still be normocapnic during the day. Therefore, nocturnal measurements of PCO2 are required to avoid overlooking the onset of chronic ventilatory failure. There are, however, no established cutoff values for nocturnal or daytime PCO2 that would reliably indicate the need for HMV. Previous international recommendations have provided PCO2 cutoff values for specific disease groups, but these recommendations are based on expert consensus rather than reliable research1; moreover, this report only refers to NPPV, thus excluding invasive mechanical ventilation. The report1 recommends long-term NPPV for restrictive patients when PaCO2 is equal to or higher than 45 mm Hg, while a PaCO2 equal to or higher than 55 mm Hg is recommended in patients with COPD. In cases where nocturnal desaturations occur, despite oxygen treatment, or in cases with recurrent hospitalizations in order to manage acute hypercapnic respiratory failure, a PaCO2 reading of 50 to 54 mm Hg warrants long-term NPPV.1 Although detailed criteria are provided in the recently published German guidelines for HMV,35 and a Canadian guideline is in preparation (personal communication), an international consensus on when to start HMV has not been reached. However, HMV needs to be carefully considered, not just in terms of PaCO2 levels. Patients should be severely symptomatic with reduced quality of life, and should also be very likely to benefit from the therapy. This is particularly important for patients with COPD in whom clear improvements in long-term survival have yet not been scientifically established.5,22,23 Nevertheless, the use of HMV for preventive purposes in nonhypercapnic patients should be avoided.38
For the commencement of HMV, chronic hypercapnic failure with normal pH (resulting from bicarbonate retention) should be established and distinguished from acute hypercapnic respiratory acidosis (Table 28-4).
| Acute | Acute on Chronic | Chronic | |
|---|---|---|---|
| pH | Lowered | Lowered | Normal |
| PaCO2 | Increased | (Greatly) increased | Increased |
| HCO3 | Normal | Increased | Increased |
In general, NPPV should be used in preference to invasive mechanical ventilation, which is used more often in neuromuscular patients following tracheostomy than in other disease groups.8 In particular, tracheostomy is indicated for the following conditions39–43: lack of mask fitting; intolerance of, or claustrophobia with, mask ventilation; ineffective ventilation; impaired bulbar function with recurrent episodes of aspiration; insufficient noninvasive management of secretions requiring suction via a tracheal cannula; and failure to wean off invasive mechanical ventilation following intubation.
It is extremely important to comprehensively inform the patient and the family members about tracheostomy and its consequences. Patients’ self-determination is a top priority, and decision making should be based not only on objective circumstances but also on the subjective perspective of the patient.
Organization
Patients receiving HMV should be managed in a specialized center that is familiar with the specific circumstances and problems occurring in such patients.35 Here, high-quality, individually customized care is paramount, and it is essential that the treatment plan can be readily adapted to any necessary changes in the ventilator mode, settings, and interface, as well as in the duration, of mechanical ventilation. In all cases where the proposed treatment is subject to alteration, the patient, together with relatives and all associated therapists, must be included in the decision-making process, which must include close consultation and good organization between all participating individuals.
It is well recognized that the organizational structures vary considerably among countries and regions.8 Preferably, a specialized unit is set up exclusively for HMV and integrated into a network of specific units qualified for treating patients with mechanical ventilation (Fig. 28-4). Patients are usually admitted to the HMV unit from different sites within the same center, or from external sites (Fig. 28-4). Because many patients have sleep-disordered breathing, the center should provide easy access to full polysomnography, preferably within an in-house sleep laboratory. Many patients, particularly those with obesity hypoventilation syndrome, are primarily admitted to a sleep laboratory for diagnosis of sleep disturbances. In this case, sleep-related hypoventilation with deteriorating hypercapnia must not be overlooked, thus requiring close monitoring of nocturnal PCO2 and the transfer to the HMV unit where appropriate.
Figure 28-4
Specialized center for home mechanical ventilation. Patients are usually transferred to the HMV unit from other units within the specialized center, such as the sleep laboratory and weaning unit (light blue), or from external facilities (white) including outpatient clinics and external, nonspecialized hospitals. Return transfer should be facilitated at all times, according to patient’s needs. ICU, intensive care unit; RICU, respiratory intensive care unit.
Especially confounding is the fact that HMV candidates are not always patients electively admitted to hospital: Some patients may also present with weaning failure consequent to ventilator management for acute respiratory failure (see Fig. 28-4). These patients are usually transferred from the intensive care unit to the HMV unit via a weaning unit (“step-down”).43 With the marked increase in the number of severely ill weaning-failure patients being admitted to a weaning unit,44 close cooperation between the weaning and the HMV units is mandatory, because many weaning-failure patients are ultimately established on HMV.43,44
Hospital staffing requirements depend on the type and severity of a patient’s underlying disorder. Staffing also relies on the local conditions of the center, such as experience, size, and country-specific organization of health care.8 Accordingly, great variation in staffing demands exists amongst different centers. Nevertheless, the global standards for staff include satisfactory training in mechanical ventilation techniques that covers both invasive and noninvasive ventilation. The team must be familiar with specific treatment modalities, such as airway clearance techniques, management of acute respiratory failure, and disease-specific issues.45,46 It is the responsibility of the center to provide sufficient staff training for all professionals involved, which may include: nurse, physician, physiotherapist, equipment provider, psychotherapist, respiratory therapist, and social worker.
In addition, a speech therapist, a nutritionist, or other therapist may be necessary at some point during the treatment of patients with HMV.
Transferring patients from hospital-based to home-based treatment is always a challenge.35,45–48 The challenge results from the necessity of replacing the in-hospital care team with an out-of-hospital care team, which may also include lay helpers, most importantly relatives. Training of all people caring for HMV patients in the home environment is essential.35,45,46 In addition, the specialized center and the provider should always be contactable for solving medical and technical problems, respectively.35,45,49
Both the underlying and secondary illnesses need to be in a stable condition before discharge.50 If an optimal level of function and performance is not yet reached, (early) rehabilitative measures should be considered.35,45 In addition, the fulfilment of costs and provision of the necessary equipment, resources, and materials need to be ascertained in advance.35 Individual requirements for patient discharge and subsequent setup of the home-ventilation station vary significantly from patient to patient. Table 28-5 is a checklist of the minimal requirements.35
|
Control visits include the collection of data on compliance, side effects, ventilation techniques, and daytime respiratory function, and also on nocturnal diagnostics, particularly nocturnal assessment of alveolar ventilation. Preferably, this should be performed in the specialized center where the patient has been initially treated. The first control examination after commencement of HMV should take place 4 to 8 weeks after discharge.35,47,51 Subsequent control visits typically occur once or twice a year and are carried out on an individual basis, depending on the type and progression of the underlying disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree