In 2001, Rivers and colleagues reported that among patients with severe sepsis or septic shock in a single urban emergency department, mortality was significantly lower among those who were treated according to a 6-hour protocol of early goal-directed therapy (EGDT) than among those who were given standard therapy (30.5% compared with 46.5% mortality).3 The EGDT protocol is shown in Figure 20-2.
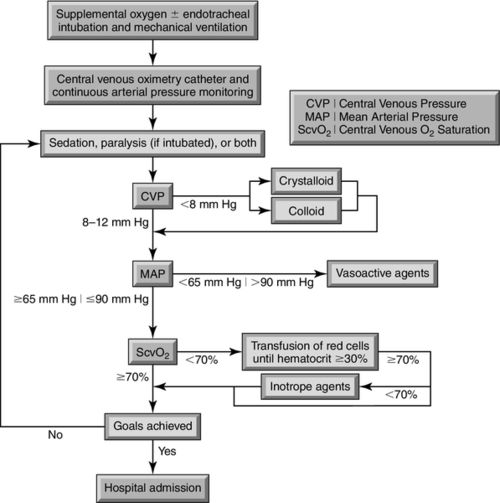
The clinical targets in this trial included achieving a central venous pressure (CVP) of greater than 8 millimeters of mercury (mm Hg) in spontaneously breathing patients or greater than 12 mm Hg in patients who were mechanically ventilated;3 maintaining mean arterial pressure (MAP) above 65 mm Hg,3 and attaining a central venous oxygen saturation (ScvO2) greater than 70%, or a mixed-venous oxygen saturation (SvO2) greater than 65%.3 Early goal-directed sepsis management has led to one of the greatest reductions in sepsis-related morbidity and mortality over the past 50 years.
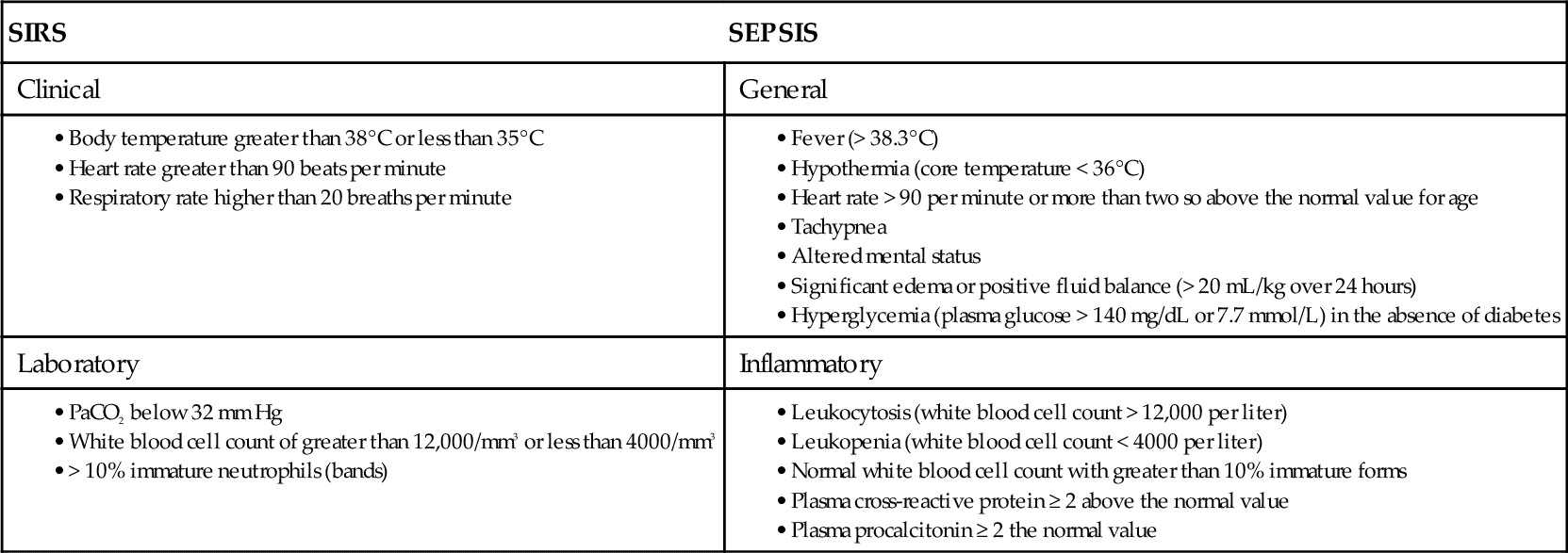
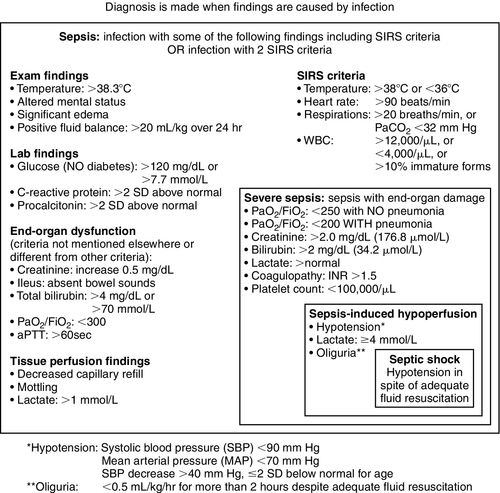
On the basis of this and other studies, the Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) created the “Surviving Sepsis Campaign” (SSC) in October 2004 with a vision of decreasing sepsis mortality by 25% over the following 5 years.4,5 One of the most important contributions of the campaign was clarification of the definitions for sepsis and for systemic inflammatory response syndrome (SIRS) as listed in Table 20-1.
The most recent guidelines update was published in 2013. Early identification, antibiotics, and source control, as well as initial volume resuscitation remain as the foundation of sepsis management.6,7 The current recommendations for the resuscitation timeframe are listed as the Surviving Sepsis Campaign bundles (Box 20-1).
For most patients with sepsis syndrome, the failure of three or more organ systems results in a mortality rate above 90%. The mortality rate for patients with acute kidney injury in the setting of sepsis ranges from 50% to 80%. Recent research and innovation has focused on the molecular mechanisms and hemodynamics of sepsis and septic shock.8
Identifying the very generic presentation of systemic inflammatory response syndrome offers little guidance for clinicians, as many disease processes present in a similar fashion. As sepsis progresses, however, the syndrome may spiral into a generalized circulatory, immune, coagulopathic, and neuroendocrine response, that ultimately promotes vascular dysfunction, failure of oxygen delivery, and inability to meet tissue oxygen consumption needs. This forms the continuum of SIRS to septic shock.
Epidemiology of Sepsis to Septic Shock
The significance of septic shock associated mortality continues to be a worldwide health issue. In the United States (U.S.) alone, 2% of all hospital admissions have a diagnosis of severe sepsis, which represents 10% of all patients admitted to critical care units.9 The incidence of severe sepsis (sepsis-induced organ dysfunction) in the European Union has been estimated at 90.4 cases per 100,000 populations, compared to 58 per 100,000 for breast cancer.10 In a comprehensive U.S. National Hospital Discharge Survey published in 2011, the incidence of sepsis-related hospitalizations increased from 221 to 337 cases per 100,000 per year between 2000 and 2008.10a Older age is a significant risk factor. The risk of a sepsis-related hospitalization for people over 85 years of age is 30 times that of people under 65 years of age.10a
Septic shock is defined as a state of acute circulatory failure characterized by persistent hypotension that is unexplained by other causes despite adequate fluid resuscitation, and its incidence is increasing. Mortality from septic shock in critical care is estimated to range between 45% and 63% in observational studies.11–14 The average sepsis survivor requires 7 to 14 days of critical care unit support with much of this time spent on a ventilator. An additional 10- to 14-day hospital stay is typical, creating an average hospital length of stay for survivors of up to 3 to 5 weeks.15 Hospital charges in excess of tens of thousands of dollars for individual patients are typical, resulting in annual expenditures of nearly $17 billion in the U.S.16 In 2009, sepsis was the sixth most frequent and the single most expensive U.S. hospital admission diagnosis. In 2011, the Agency for Healthcare Research and Quality (AHRQ) listed sepsis as the most expensive condition treated in U.S. hospitals, costing more than $20 billion in that year.7 One estimate is that treatment of sepsis costs $44,600 for the initial admission alone, with costs increasing dramatically with prolonged hospital stays and any complications arising from the sepsis sequelae.17
Sepsis Pathophysiology
In its most fundamental presentation, sepsis is a clinical syndrome characterized by SIRS, or a presumed or documented infection and tissue injury. It represents a continuum of severity that results in a cascade of biochemical and pathophysiologic events. If left unabated, microbial toxins together with a dysfunctional host immune response quickly lead to tissue damage, shock, organ failure, and death.
The major pathways involved in sepsis include the innate immune response, inflammatory cascades (cytokine and arachidonic), procoagulant and antifibrinolytic pathways, alterations in cellular metabolism and signaling, as well as an ultimate acquired immune dysfunction. The pathways and their effects are shown in Figure 20-3.18–21
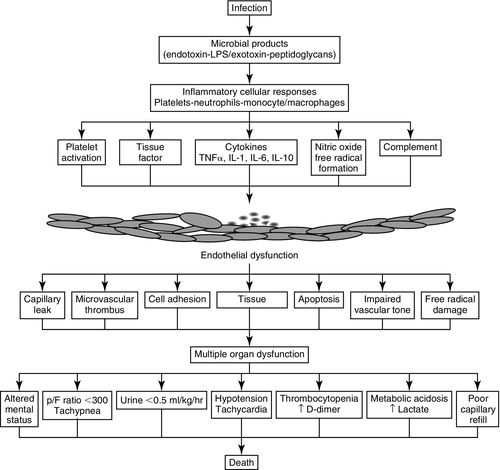
Sepsis is characterized by the cardinal signs of inflammation (vasodilation, leukocyte accumulation, increased microvascular permeability) occurring in tissues that are remote from the site of stimulation along with the suspicion or confirmation of infection. In certain patients, the normal innate immune response, stimulated by invading pathogens may augment the cascade of sepsis pathophysiology. Cytokines regulate multiple inflammatory responses, including localizing and controlling infection. Deregulated cytokine release may lead to endothelial dysfunction, vasodilation, and increased capillary permeability. The resulting cellular leakage syndrome disrupts regulatory mechanisms and leads to profound intravascular hypovolemia, cellular dysfunction, and, ultimately, cell death. Hemodynamic alterations in sepsis are directly related to endothelial derangements, tissue hypoxia, mitochondrial dysfunction, decreased oxygen delivery, and alterations in blood flow. 22
The ultimate result may be systemic vasodilation, heart failure, and microcoagulopathy, all contributing to a significant alteration of microvascular blood flow, decreased oxygen delivery, and profound dysoxia at the mitochondrial level.23
In addition, circulating or expressed mediators promote a loss of arterial vasomotor tone and myocardial depression. Cardiac output cannot increase because of the myocardial depression; nor can the stroke volume maintain a constant driving blood flow in the face of the dilated vasculature, which is presented as refractory hypotension. This cascade of events is partly responsible for the imbalance between systemic oxygen delivery and oxygen demand, leading to global tissue hypoxia.
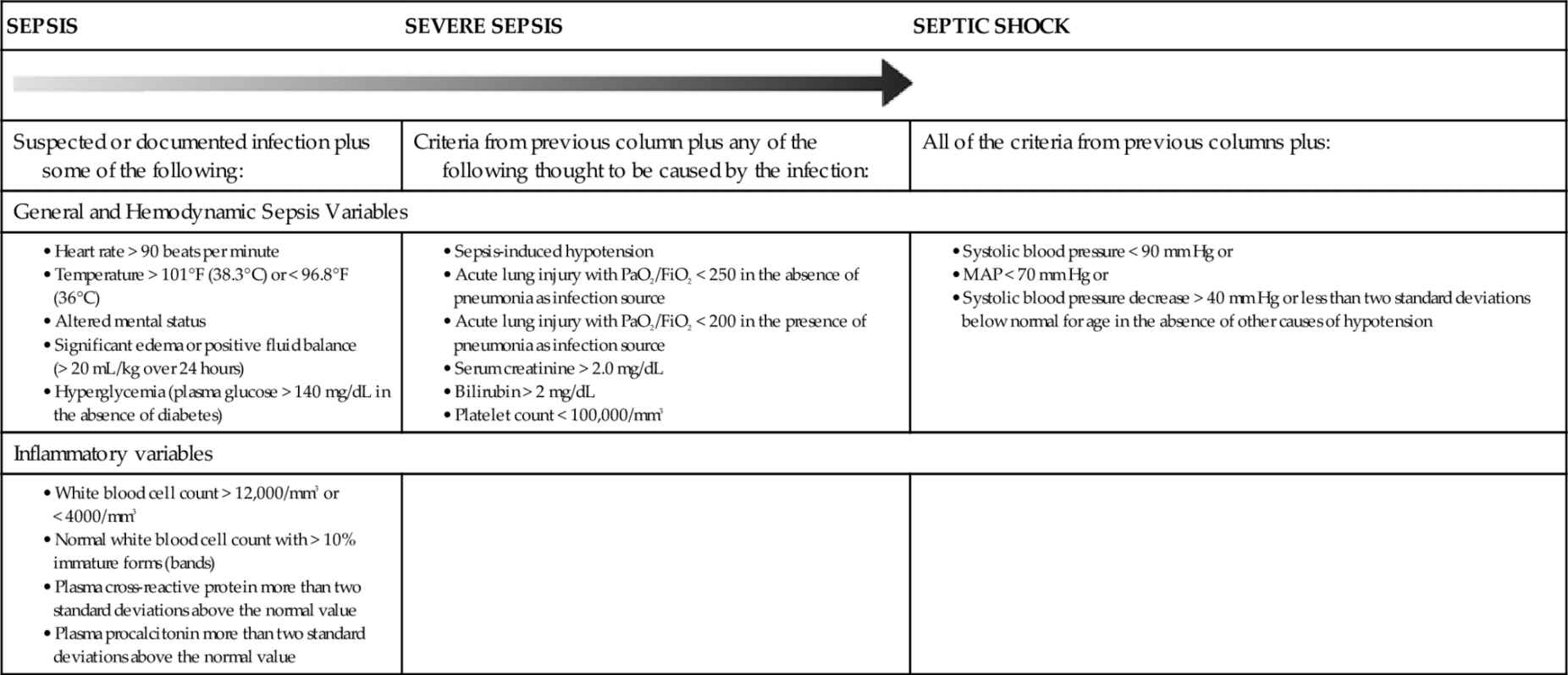
Severe sepsis and septic shock are characterized at different points on a continuum and integrate signs and symptoms of hypovolemic shock, cardiogenic shock, and distributive shock. Patients with severe sepsis and septic shock often exhibit all three states simultaneously, making effective diagnosis and therapeutic interventions difficult to standardize and achieve. In clinical practice, the terms sepsis, severe sepsis, and septic shock are generally used interchangeably to describe a myriad of events along this continuum of presentations.
Sepsis Definitions
The diagnosis of sepsis requires clinical evidence of infection and the presence of SIRS, which is characterized by specific physiologic alterations, including changes in temperature, white blood cell count, heart rate, and respiratory rate.24,25
The terms SIRS, sepsis, and severe sepsis, and septic shock are not interchangeable, although often presented as such. The definitions are discussed in more detail below. These definitions drive the interventions, as recommended in the Surviving Sepsis Campaign guidelines.6,22
Systemic Inflammatory Response Syndrome
SIRS may result from numerous conditions but is only defined as sepsis when the etiology includes infection (Figure 20-1). SIRS presents as a deregulated inflammatory response, regardless of origin, when two or more clinical signs are present. The salient signs of SIRS and sepsis are listed in Table 20-1. This SIRS response has been criticized for a lack of specificity for sepsis because the signs represent a clinical response to acute inflammation.26 The pathogenesis of SIRS may be associated with the release of bacterial toxins and cellular mediators that cause systemic instability.27
Sepsis
Sepsis is defined as infection accompanied by two or more of the following signs of systemic inflammation: hypothermia or hyperthermia, tachycardia, tachypnea, and elevated or depressed leukocyte count. The laboratory diagnosis may be supported by positive cultures, tissue stain, or polymerase chain reaction tests as listed in Table 20-2.
Procalcitonin
The utilization of the peptide procalcitonin, which is released in response to proinflammatory stimuli, is a biomarker for infection and may have a higher diagnostic accuracy compared with other traditional biomarkers of inflammation.29,30 The findings of several meta-analysis present conflicting evidence as to the benefits of procalcitonin levels and early recognition of sepsis.31–34
In general, evidence supports procalcitonin as more helpful for making individualized decisions regarding antibiotic stewardship, including discontinuation and withholding of antibiotics. The initiation of antibiotics in the presence of hypotension remains the single-most important therapeutic intervention for improving outcomes of patients with sepsis.35
Severe Sepsis
Severe sepsis requires the presence of at least one new organ dysfunction. Or an acute injury may be superimposed on chronic organ dysfunction.
Septic Shock
Septic shock is defined as hypotension with organ system dysfunction that is not correctable by intravenous fluid resuscitation and requires the addition of vasopressors. Shock is a clinical diagnosis defined by inadequate end-organ perfusion in the setting of hemodynamic instability (Figure 20-4).
Hemodynamics of Sepsis: Variability in Oxygen Delivery, Vascular Tone, and Arterial Volume
The cardiovascular and endothelial systems play a key role in the pathophysiology of severe sepsis and septic shock, and both are central and critical. Distinguishing between the direct effects of sepsis on the myocardium, endothelial alterations, and cardiac responses to changes in preload, afterload, and neurohumoral activity is challenging.
Hemodynamic monitoring endpoints for therapeutic interventions focus on five primary areas:
Preload: Volume Status in Severe Sepsis and Septic Shock
Determining the optimal preload level in sepsis is challenging. A patient who is not considered fluid responsive may be potentially harmed by aggressive fluid resuscitation. In fact, in non–fluid responsive patients, the volume may exacerbate acute respiratory distress syndrome (ARDS), acute kidney injury, and intra-abdominal hypertension. Traditional methods for monitoring, namely the CVP, and pulmonary artery occlusion pressure (PAOP), were used to gauge volume resuscitation, however, the utility of these methods is now considered dubious even when high or low. CVP and PAOP are considered static hemodynamic measurements, as they are obtained under a single ventricular loading condition.
Central Venous Pressure
The CVP is used to obtain a static measurement of preload. The validity of CVP measurement in patients with severe sepsis or septic shock is widely debated. There is no known threshold value of CVP that identifies patients whose cardiac output will increase in response to fluid resuscitation.36 Recent evidence negates the value of the CVP-driven protocol endpoint in the Surviving Sepsis Campaign guidelines, as new findings suggest that fluid management utilizing CVP-targeted resuscitation contributes significantly to morbidity and mortality.37–39
The Surviving Sepsis Campaign both acknowledges the limitations of CVP monitoring and accepts that a low CVP generally indicates low intravascular volume although there has been evidence to the contrary. Current Surviving Sepsis Campaign guidelines recommend fluid resuscitation of patients presenting with sepsis-induced tissue hypoperfusion to a targeted CVP of 8 to 12 mm Hg (or 12 to 15 mm Hg in mechanically ventilated patients) within 6 hours of sepsis identification. The rationale behind this CVP-targeted fluid resuscitation is to ensure adequate cardiac preload and maintain cardiac output and organ perfusion. However, absolute levels or changes in CVP poorly predict cardiovascular response to volume resuscitation or fluid responsiveness.40
Pulmonary Artery Catheter
The pulmonary artery catheter (PAC) measures or calculates intrapulmonary pressures, cardiac output, and, on particular types of PACs, mixed venous oxygen saturation (SvO2). A systematic review of the use of PAC catheters, which included 13 studies and 5686 patients, revealed that the use of the PAC did not alter mortality, or critical care unit length of stay of these patients.19 The PAC-Man (Pulmonary Artery Catheters in Patient Management) trial, performed between 2001 and 2004, provided the strongest evidence to date that PAC use conferred neither benefit nor harm to critical care patients.41 Consequently, a PAC is not routinely inserted for volume guidance in sepsis management.
Dynamic Measures of Fluid Responsiveness
Today, dynamic or volumetric measures may offer a better guide to fluid responsiveness. This indicator is evaluated across the thoracic inspiratory and expiratory pressure changes that affect the volume loading and compliance of the right ventricle immediately and the left ventricle thereafter, related to the pulmonary transit of blood.
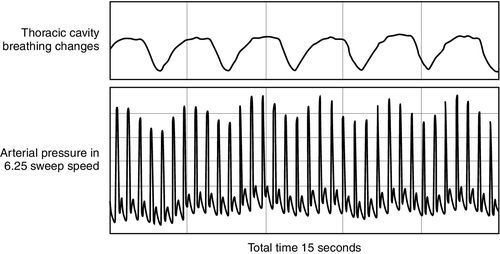
Heart–lung interactions during mechanical ventilation are used to evaluate the variations in stroke volume, systolic pressure, and pulse pressure. Pulse pressure variation estimated from the arterial waveform, stroke volume variations from pulse contour analysis, pulse oximeter plethysmographic waveform, and thoracic bioreactance have been found to be reliable predictors of fluid responsiveness when patients receive a volume challenge test such as a passive leg raise.42,43
Multiple methods exist for evaluating a volume challenge. It is possible to view arterial waveform variability with the bedside monitor by setting the sweep speed for the arterial pressure at 6.25 millimeters per second (mm/sec), allowing for a more compressed time view as shown in Figure 20-5. Although quantifying the variability can be somewhat difficult, the presence of variability is helpful when observed.
Other ways to evaluate volume responsiveness or variability are to utilize minimally invasive, arterial-based stroke volume variation (SVV), or arterial-based pulse pressure variation (PPV). These techniques, measured via specialized arterial transducers or bioreactance electrodes, correlate volume changes to positive pressure breathing, which increases intrathoracic pressure during inspiration in a predictable pattern. Minimally invasive, arterial-based systolic pressure variation (SPV) can be measured on any patient regardless of ventilation status.44,45 The impact of mechanical ventilation on hemodynamic monitoring is discussed in detail in Chapter 14.
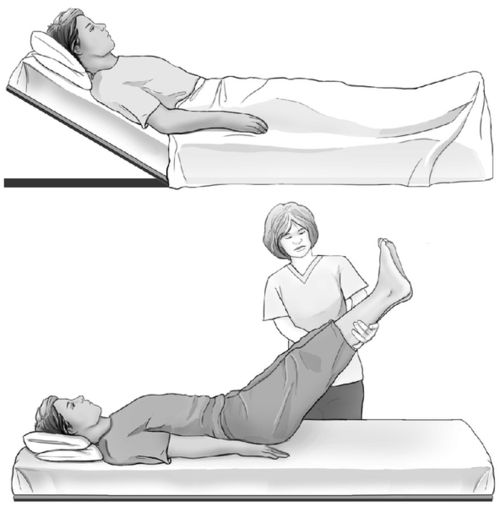
Volume responsiveness can be assessed noninvasively with plethysmography variation, to generate a perfusion index (Philipps) or a perfusion variability index (Massimo). In combination with one of these measurement technologies, the passive leg raise can help to determine whether or not the patient is likely to be fluid responsive (Figure 20-6).46,47 More information about the passive leg raise maneuver is presented in Chapters 3 and 12.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree