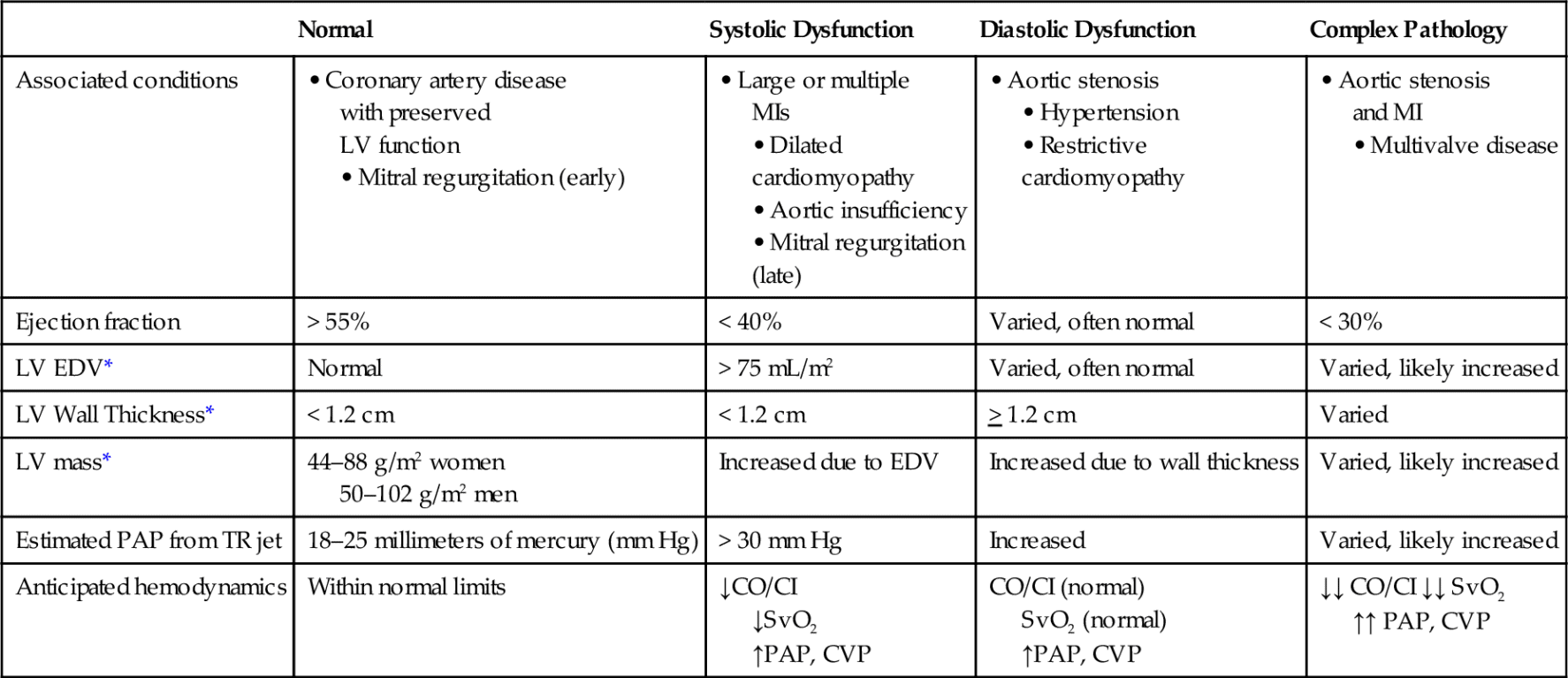
On admission of the patient to the critical care unit, clinicians should anticipate that the patient with a stable hemodynamic profile will look well perfused, produce urine in large amounts, and exhibit hemodynamic data that are either within normal limits or promptly trending in that direction. Should this patient unexpectedly develop a low cardiac index or SvO2 and elevated filling pressures, clinicians are alerted to immediately explore potential causes such as ischemia or tamponade. Either of these conditions may present with sudden cardiac compromise or even arrest. A more typical scenario is a gradual yet persistent decline in blood pressure, cardiac index, and SvO2 in combination with compensatory increases in heart rate and SVR. This signals an abnormal trajectory that warrants further assessment. In contrast, patients with either systolic or diastolic dysfunction are expected to display substantially different postoperative presentations and “normal” hemodynamic parameters may not be achievable, as illustrated in Table 16-1. Distinguishing these clinical profiles is essential to developing appropriate postoperative goals and devising optimal strategies to achieve them.
Table 16-1
Predicted Hemodynamic Profiles Based on Preoperative Diagnostic Data
Data from Otto CM: Textbook of clinical echocardiography, ed 5, Philadelphia, 2013, Saunders.
* Adjusted for body size. CI, Cardiac index; CO, cardiac output; cm, centimeters; CVP, central venous pressure; EDV, end-diastolic volume; g/m2, grams per square meter; LV, left ventricle; MI, myocardial infarction; mL/m2, millimeters per square meter; PAP, pulmonary artery pressure; SvO2, mixed venous oxygen saturation; TR, tricuspid regurgitation.
Hemodynamics in Systolic Dysfunction: The Volume Overloaded Ventricle
Chronic impairment of systolic function, often accompanying myocardial infarction (MI) or mitral valve regurgitation, leads to an “overstretched” left ventricle and is associated with chamber dilation with reliance on volume loading for optimal contractility (Figure 16-2). Preoperative findings indicative of moderate to severe systolic dysfunction should influence the clinician’s estimation of appropriate hemodynamic goals postoperatively. Ejection fraction is a useful gauge of anticipated LV performance; marked reductions in ejection fraction equate to the potential for greater hemodynamic aberrations postoperatively because cardiac output is lower, and filling pressures are higher than normal. Additional findings of systolic dysfunction include increased pulmonary artery pressures and end-diastolic volume, as well as LV mass, which is augmented by increased volume, not wall thickness (see Table 16-1).11
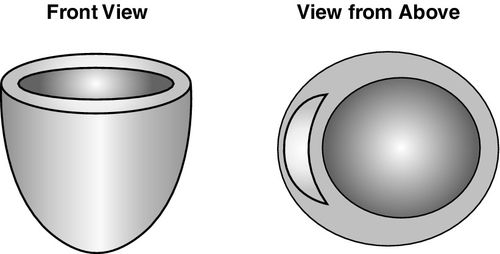
The impact of corrective surgery on mitral regurgitation merits special mention, as the procedure eliminates an incompetent mitral valve that was providing a potentially important compensatory release mechanism in the presence of increased LV afterload. Following mitral valve repair or replacement, the blood volume must now correctly exit via the high-pressure forward outflow tract of the left ventricle, placing increased demands on chamber. Postoperatively, this increased afterload typically leads to reduced ventricular performance and a lower ejection fraction. Thus, preoperative ejection fraction measurements will overestimate ventricular function in patients with mitral regurgitation.12 Patients with severe LV contractile dysfunction may be incapable of overcoming even a modest afterload increase without marked pharmacologic and mechanical circulatory support, but this is not always discernible from the preoperative ejection fraction. For this reason, the highest mortality risk is seen in patients undergoing replacement of the mitral valve, particularly if combined with coronary artery bypass or aortic valve replacement.8
With chronic volume overload, adequate fluid resuscitation is critically important to optimize systolic function. However, filling pressures become a less sensitive indicator of fluid status in the dilated left ventricle that is accustomed to a large end-diastolic volume. The thin-walled, compliant heart accommodates large increases in chamber volume, yet hemodynamic monitoring reveals only minimal pressure changes. This is reflected in an abnormal ventricular compliance curve that is relatively low and flat, with a late upstroke in pressure values as shown in Figure 16-1. Absolute numbers are less helpful in determining fluid status than monitoring the hemodynamic response to volume loading: if cardiac performance increases after a fluid bolus, the patient can be considered “volume responsive” regardless of the filling pressures.7
Rather than trying to achieve “normal” parameters for patients with LV dysfunction, fluids, medications, and cardiac support devices are titrated to achieve values more realistically aligned to systolic capability and hemodynamic trends. Goals for blood pressure and cardiac index are lower, and those for filling pressures are higher than normal, with exact endpoints targeted initially to intraoperative values that achieved optimal performance. Use of vasodilators and phosphodiesterase inhibitors to reduce afterload may benefit the poorly contracting left ventricle but must be cautiously titrated to avoid causing hypotension. Heart rate is a significant contributor to cardiac index, and the use of temporary pacing to maintain atrioventricular synchrony at a rate of 90 to 110 beats/min may offer hemodynamic benefits for patients with postoperative ventricular dysfunction.
Determination of acceptable hemodynamic targets, particularly when outside of normal ranges, must be clarified with all providers and adjusted frequently based on predetermined hemodynamic endpoints and clinical response. Strategies should achieve continued steady progress toward individualized goals while avoiding rapid rewarming or abrupt adjustments that could lead to acute deterioration of LV function or precipitate arrhythmias. In patients who are incapable of increasing their cardiac output, even modest increases in oxygen demand may precipitate decompensation. These patients derive benefit from frequent reassessment of continuously monitored variables, including cardiac output, cardiac index, and SvO2. This is to enable careful titration of therapies and facilitate prompt detection of potentially destabilizing conditions such as hypoxia, shivering, seizures, or occult bleeding. A sudden decline in the SvO2 signals an important, often occult, imbalance between global oxygen supply and demand that warrants immediate assessment and intervention. In a large Swedish trial of coronary artery bypass surgical patients (n = 2755), the mean SvO2 on arrival to the critical care unit was 66% and values less than 60% were associated with a fivefold increase in early mortality.6 In patients with aortic stenosis, marked reductions in survival were seen when the initial SvO2 was less than 55%.13
Hemodynamics in Diastolic Dysfunction: The Pressure Overloaded Ventricle
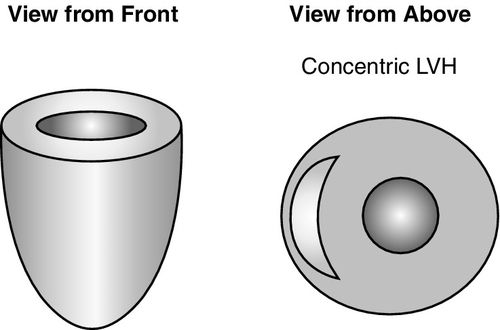
Chronic hypertension, hypertrophic cardiomyopathy, and aortic stenosis are conditions that result in increased LV afterload. With obstruction to LV outflow, hypertrophy of the cardiomyocytes occurs in an attempt to overcome resistive forces to ejection, resulting in increased LV mass (Figure 16-3). The degree of hypertrophy is a significant risk factor for adverse outcomes and has a strong influence on hemodynamic goals.14 The thickened left ventricle can easily generate an adequate systole, but impaired diastolic function and failure to relax contribute to underfilling, which is substantially worsened by atrial fibrillation and loss of atrial kick, or inadequate fluid resuscitation. Echocardiographic findings of diastolic dysfunction include elevated pulmonary artery pressures, greater wall thickness, and LV mass caused by increased muscle mass versus volume overload. Ejection fraction and end-diastolic volume are typically within normal limits in these patients but may be reduced to a modest degree.11

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








