DEFINITION
Hypotension is broadly defined as abnormally low blood pressure. Investigators have often developed operational definitions of hypotension for use in individual studies. It is the variations in these operational definitions that often lead to confusion and inconsistencies when comparing different studies. One common operational definition of hypotension is any systolic blood pressure below a predetermined level, usually 80 or 90 mm Hg. Alternatively, a fixed percentage reduction (commonly 30%) in systolic or mean blood pressure from that patient’s baseline can also be considered hypotension. Finally, some studies classify hypotension as a rapid rate of decline in blood pressure (such as 30% or 30 mm Hg over 5 minutes) regardless of the absolute value. The mechanisms involved in this latter definition of hypotension may be different from those involved in slower rates of decline. Furthermore, the method by which blood pressure is measured is essential to understanding the occurrence of hypotension in any study. Invasive measurements versus noninvasive measurements should be specified, as well as the level at which the transducer is placed, or the location at which the noninvasive blood pressure cuff is situated. These variables may all impact the interpretation of hypotension.
Bradycardia can be broadly defined as an abnormally low heart rate. This complication can also be described operationally as a heart rate below a fixed level (50 beats per minute, bpm), a percentage reduction from baseline (30%), or a sudden decrease at a predetermined rate. Again, the mechanisms involved in a slow decline in heart rate over the course of a regional anesthetic may be very different from a sudden drop in heart rate over 1 to 2 minutes (Fig. 6-1). Although the complications of hypotension and bradycardia are most often associated with neuraxial anesthesia, these hemodynamic changes have also been described during shoulder arthroscopy in the sitting position under interscalene block.1 Although it may seem as though this setting has little in common with spinal or epidural anesthesia, some mutual hemodynamic conditions exist that make the pathophysiological origin of these events related.
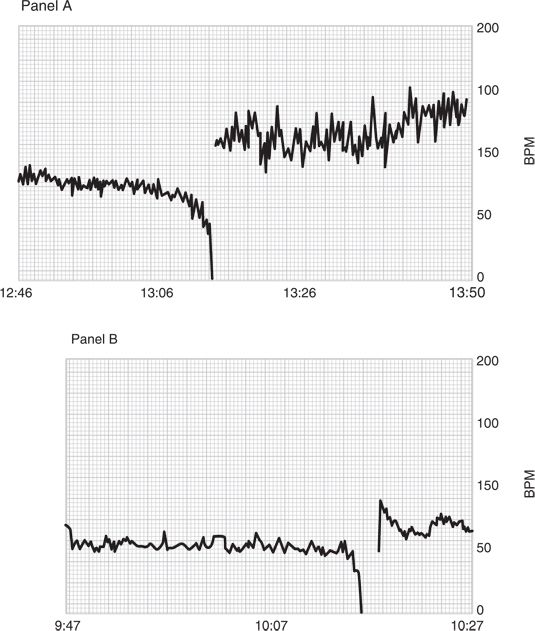
FIGURE 6-1. An example of a slow decline in heart rate (panel A) versus a sudden drop (panel B).
 SCOPE OF THE PROBLEM
SCOPE OF THE PROBLEM
The incidence of hypotension and bradycardia in patients undergoing neuraxial anesthesia is quite significant. Several large-scale reports place the incidence of bradycardia between 2% and 13%. The incidence of hypotension in these same studies ranges from 8% to 33% (Table 6-1). Although the incidence of these hemodynamic complications is relatively high, the medical significance of the events is variable. Quite often, these occurrences are ignored or easily treated or in some cases they are desirable hemodynamic conditions2 for a given operation.
TABLE 6-1 Reported Incidence of Hypotension and Bradycardia Associated with Neuraxial Anesthesia
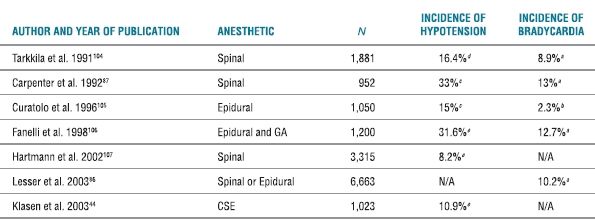
aHR < 50.
bHR < 45.
cSBP < 90.
dA 30% decrease in SBP from baseline or SBP < 85.
eA 30% decrease in SBP from baseline.
GA, general anesthesia; SBP, systolic blood pressure; CSE, combined spinal epidural; and N/A, not applicable.
Severe hypotension and bradycardia leading to cardiac arrest may also occur with regional anesthesia. An early case in the anesthesia literature of a cardiac arrest following a regional anesthetic was reported by Wetstone and Wang in 1974.3 The patient was a healthy 29-year-old male undergoing a minor urologic procedure under spinal anesthesia. The arrest was successfully treated with atropine and closed-chest cardiac massage, with no long-term sequelae. Over a decade later, the issue of sudden unexpected cardiac arrest under spinal anesthesia was again highlighted in the anesthesia literature. Caplan et al.’s closed-claims analysis in 19884 described 14 cases of cardiac arrest under spinal anesthesia in healthy patients. Half of the cases were possibly attributable to respiratory events. The remainder were likely of a primary circulatory etiology. The majority of patients in this report had poor outcomes. A subsequent report in 19895 described three cardiac arrests during spinal anesthesia that were not respiratory in nature, as evidenced by oxygen saturation immediately prior to the arrest remaining normal. This finding was confirmed in multiple other case reports involving both spinal6–10 and epidural11,12 anesthetics. It is now clear that cardiac arrests may be precipitated primarily by nonhypoxic circulatory conditions during a neuraxial anesthetic.
Several large-scale studies have attempted to determine the incidence of cardiac arrest under regional anesthesia (Table 6-2). Although the rate of occurrence of cardiac arrests during regional anesthesia is low, the mortality associated specifically with spinal anesthesia remains exceedingly high. In one report, cardiac arrest secondary to a neuraxial block accounted for the largest category of malpractice claims associated with death or brain damage.13
TABLE 6-2 Incidence of Cardiac Arrests (and Death) Associated with Regional Anesthesia
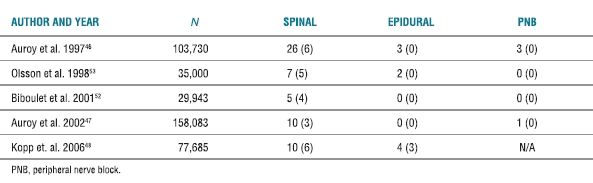
PNB, peripheral nerve block.
Sudden severe hypotension and bradycardia have also been noted during shoulder surgery under interscalene block in the sitting position. Several prospective and retrospective studies have confirmed that these events may occur in 13% to 29% of cases (Table 6-3). Although acute hypotension and bradycardia do occur with significant frequency, serious morbidities or mortality have yet to be reported in this scenario.
TABLE 6-3 Incidence of Hypotensive/Bradycardic Events Associated with Shoulder Arthroscopy in the Sitting Position Under Interscalene Block
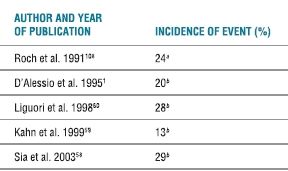
Definition of event: A decrease in HR of >30 BPM in <5 min or any decrease to <50 BPM and/or a decrease in SBP of >30 mm Hg in <5 min or any decrease to <90 mm Hg.
aEpinephrine added to ISB and beta blocker use not addressed.
bEpinephrine added to ISB and no beta blockers given.
A related situation that has recently been described is the occurrence of cerebrovascular events during shoulder surgery in the sitting position utilizing intentional hypotension to minimize blood loss.14 Since only case reports are available, the incidence of these events is unknown. Given the likelihood that the incidence of these events is extremely rare, large-scale studies will be required to evaluate their occurrence. It is clear, however, that the morbidity during these events has the potential to be catestrophic. Using near-infrared spectroscopy, Murphy et al. described an 80% incidence of cerebral desaturation events in the sitting position (compared with 0% in the lateral position) for patients undergoing shoulder arthroscopy.15 None of these patients, however, developed any significant anesthestic morbidities.
 PATHOPHYSIOLOGY
PATHOPHYSIOLOGY
Neuraxial Regional Anesthesia
The primary determinant of hypotension induced by spinal or epidural anesthesia is sympathetic nerve blockade.16 The greater the extent of sympathetic block achieved, the more hypotensive the patient may become. These sympathetically induced effects on blood pressure are mediated through changes in systemic vascular resistance and cardiac output. By inhibiting sympathetic neuronal output to resistance and capacitance vessels,17,18 systemic vascular resistance and cardiac output are both decreased. This is mediated through a shift in blood volume from the heart and thorax to the mesentery, kidneys, and lower extremities.19 The mean arterial pressure, in turn, falls (Fig. 6-2). Interestingly, in one study on elderly patients with cardiac disease, ejection fraction increased significantly during spinal anesthesia,19 indicating enhanced cardiac performance and contractility in this setting.
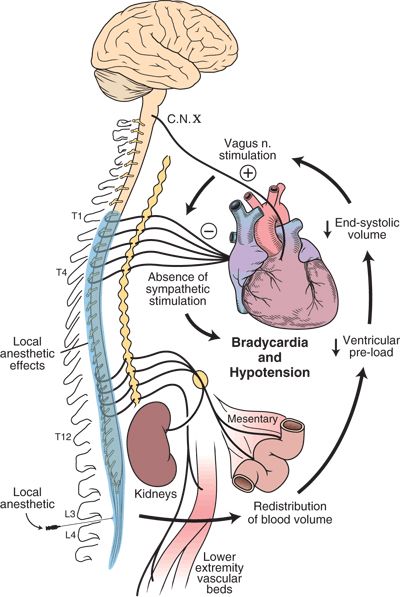
FIGURE 6-2. Pathophysiology of bradycardia and hypotension during neuraxial anesthesia.  = positive feedback
= positive feedback  = negative feedback.
= negative feedback.
Low sensory levels of neuraxial anesthesia (below T5) often result in minimal hemodynamic changes. Vasoconstriction above the level of the block often compensates for the vasodilatory effects below the level of blockade.20–23 The measurement of sympathetic level achieved during a neuraxial anesthetic is not straightforward. Several studies24,25 have attempted to relate the level of sympathetic blockade to the sensory level achieved. It is clear that predicting the degree of sympathetic block does not parallel the sensory or motor block achieved during spinal or epidural anesthesia. Early studies reported that neuraxial anesthesia had little, if any, effect on heart rate if the sensory level was below the fifth thoracic dermatome.26 This response may be partially influenced by the cardioaccelerator fibers originating from the first through fourth thoracic spinal levels (Fig. 6-2). It is now clear that the heart rate often decreases during neuraxial blockade involving lower dermatomal levels as well. As noted, the sensory level often underestimates the level of sympathetic blockade.24 Therefore, hemodynamically significant hypotension and bradycardia may occur at any time during the course or resolution of neuraxial anesthesia. Case series have given support to this theory.12
Another mechanism that explains how spinal or epidural anesthesia may induce hypotension or bradycardia is via the systemic effects of local anesthetics or vasoactive additives used during the course of an anesthetic. Local anesthetics have direct effects on both the myocardium and smooth muscle of the peripheral vasculature. All local anesthetics cause a dose-dependent negative inotropic effect on cardiac muscle.26,27 These effects are usually only observed at systemic concentrations that are higher than those attained in normal clinical settings. Furthermore, the more potent local anesthetics (e.g., tetracaine or bupivacaine) cause a greater degree of myocardial depression than less potent drugs (e.g., lidocaine or mepivacaine).26
Two common additives to local anesthetics used in regional anesthesia that may affect hemodynamic variables are epinephrine and clonidine. Epinephrine is often added to local anesthetic injections to decrease the rate of vascular absorption, increase the duration of action, or act as an intravascular marker. Absorbed epinephrine has been reported to cause a decrease in blood pressure due to β2 adrenergic stimulation.23 However, hypotension seen with epidural injection of local anesthetic and epinephrine may also be secondary to a more profound degree of sympathetic blockade.26 Clonidine induces hypotension through stimulation of α2 adrenergic receptors. Neuraxial administration causes hemodynamic effects by systemic absorption to central and peripheral sites, as well as direct effects on the spinal cord. Therefore, the degree of hypotension may be greater with thoracic epidural use compared to lumbar injection, due to the local density of sympathetic preganglionic neurons in the thoracic region. Clonidine also reduces the heart rate by presynaptic inhibition of norepinephrine release and by direct parasympathomimetic effects. These hemodynamic effects occur within 30 minutes and last from 6 to 8 hours.28
Decreased cardiac output and systemic vascular resistance caused by sympathetic blockade or direct effects of local anesthetics or additives are reasonable explanations for the slow, progressive decreases in heart rate and blood pressure observed during neuraxial anesthetics. However, sudden acute events require an additional mechanism of action to explain their occurrence (Box 6-1). Nonsurgical patients who experience sudden severe hypotension and bradycardia resulting in syncope can be evaluated by tilt-table testing. This test is based on the observation that syncope is associated with the upright posture and that concentrations of circulating catecholamines increase before the onset of symptoms.29 Therefore, syncope can be precipitated by placing a patient on a 60-degree upright tilt table and administering exogenous catecholamines.30 This response has arguably been attributed to the Bezold-Jarisch reflex.31 Stimulation of this reflex increases parasympathetic activity and inhibits sympathetic activity, resulting in bradycardia, vasodilatation, and hypotension. The hemodynamic conditions required for activation of these reflex events are a poorly filled ventricle (caused by the upright posture)32,33 and vigorous myocardial contractility (caused by endogenous or exogenous catecholamines), which in turn stimulates mechanoreceptors within the ventricular walls32. An intact system of vagal afferent fibers is required for mediation of the reflex.31
BOX 6-1 Differences in Hypotension and Bradycardia Associated with Regional Anesthesia
Gradual onset caused by sympathetic blockade secondary to:
 Local anesthetic effects
Local anesthetic effects
 Epinephrine or clonidine additives
Epinephrine or clonidine additives
Sudden onset caused by intracardiac reflex secondary to:
 Upright posture (in shoulder surgery patients)
Upright posture (in shoulder surgery patients)
 Reduced preload
Reduced preload
 Increased circulating catecholamines leading to vigorous myocardial contractility
Increased circulating catecholamines leading to vigorous myocardial contractility
During the course of a neuraxial anesthetic, a complex redistribution of blood volume occurs between the heart and various circulatory beds.34 The result of this redistribution of blood volume to the mesentery, kidneys, and lower extremities is a poorly filled ventricle with enhanced contractility.19 Therefore, the hemodynamic conditions described during tilt-table-inducible syncope are paralleled. This hypothesis was demonstrated in patients developing presyncopal symptoms during epidural anesthesia.35 Echocardiography performed in these patients confirmed reductions in central blood volume and left ventricular diameters. Furthermore, acute bradycardia and hypotension during spinal anesthesia were associated with a sudden increase in vagal activity as measured by heart rate variability analysis.36
Peripheral Regional Anesthesia
Less intuitively, shoulder surgery under interscalene block may also be associated with a decrease in left ventricular volume caused by the upright posture of the sitting position. Furthermore, enhanced cardiac contractility may occur by the addition of epinephrine to the local anesthetic or arthroscopic irrigating solution (Fig. 6-3). These hemodynamic conditions during shoulder surgery have yet to be confirmed by direct measurements in a prospective evaluation.
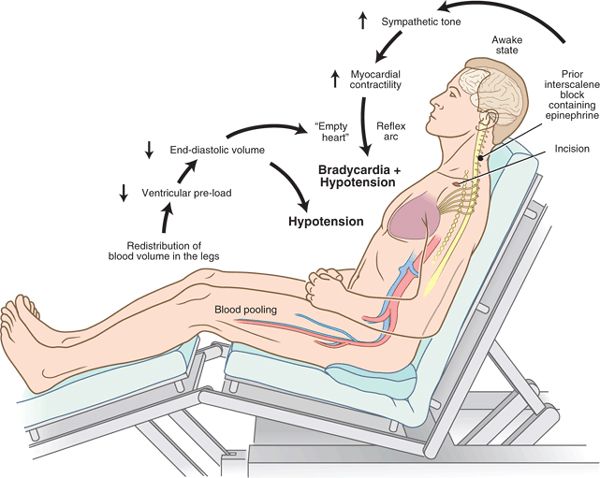
FIGURE 6-3. Pathophysiology of bradycardia and hypotension during shoulder surgery in the sitting position under interscalene block.
There is considerable controversy in the literature regarding whether these mechanisms truly explain all vasodepressor-mediated syncope33,37–39 or the potential relationships to sudden severe hypotension and bradycardia seen with regional anesthesia.40 One explanation for some of the controversy surrounding this mechanism is that activation of the Bezold-Jarisch reflex may be affected by the relative contributions of central volume depletion to the degree of fractional myocardial shortening. In other words, a vigorously contracting moderately empty ventricle may be as prone to slowing as a normally contracting severely depleted one. Another likely explanation for the contradictions in the cited studies is that this reflex arc is one of many intracardiac reflexes whose final common pathway is sudden severe hypotension and bradycardia.41,42 In any case, it is clear that a poorly filled vigorously contracting heart with an intact vagal system is prone to sudden severe bradycardia and hypotension.
The pathophysiology of the cases of cerebral ischemia during shoulder surgery in the upright position remains unclear. A proposed mechanism may be a reduction of cerebral blood flow secondary to relative intraoperative postural hypotension.14 However, each of the four cases reported involved general anesthesia. Therefore, there is no direct relationship between these events and regional anesthesia per se. In fact, a recent retrospective review of 4169 shoulder operations performed in the sitting position under interscalene block revealed a zero incidence of overt stroke in the setting of induced hypotension.43
 RISK FACTORS
RISK FACTORS
Neuraxial Regional Anesthesia
Predicting which patient or anesthetic characteristics may predispose to hypotension or bradycardia during regional anesthesia is a difficult task (Box 6-2). Several large-scale studies have attempted to evaluate various risk factors (Table 6-4). Common themes among the six studies that evaluated the risk of developing hypotension include a higher sensory anesthetic level (five studies), elevated body mass index (three studies), older patients (two studies), and the addition of general anesthesia (two studies). One study noted an increased incidence of hypotension in patients receiving combined spinal-epidural anesthesia compared with spinal anesthesia alone.44 Interestingly, Smiley et al. found evidence that genetic variations in the beta adrenoreceptor affected the incidence of hypotension during cesarean delivery.45 The common characteristics among the five studies that attempted to predict the risk factors of bradycardia include higher dermatomal level (two studies), beta-blocker use (two studies), younger patients (two studies), and lower baseline heart rate (two studies). It is clear from this summary that definitive predictive factors for the development of hypotension or bradycardia remain speculative.
BOX 6-2 Risk Factors for Hypotension, Bradycardia, and Cardiac Arrest Associated with Neuraxial Regional anesthesia
Hypotension
 High sensory block level
High sensory block level
 Elevated body mass index
Elevated body mass index
 Elderly patients
Elderly patients
 Combined regional/general anesthesia
Combined regional/general anesthesia
Bradycardia
 High sensory block level
High sensory block level
 Beta blocker use
Beta blocker use
 Younger patients
Younger patients
 Baseline bradycardia
Baseline bradycardia
Cardiac arrest
 Spinal anesthesia more frequent than epidural anesthesia or peripheral nerve block
Spinal anesthesia more frequent than epidural anesthesia or peripheral nerve block
 Development of bradycardia
Development of bradycardia
 Hip arthroplasty
Hip arthroplasty
 Elderly patients
Elderly patients
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


