TOPICS
INTRODUCTION
The management of pregnant women with hematologic disorders or coagulopathy requires a multidisciplinary approach involving hematologists, obstetricians, anesthesiologists, and possibly blood banks. Management of these patients requires the recognition of intrinsic or acquired hematologic disease in pregnancy, knowledge of the pharmacokinetics of antithrombotic medication, and an individualized approach to prevention of blood loss and use of blood products, if necessary, in the peripartum period.
COAGULATION TESTS
The evaluation of coagulation test results during pregnancy must be in the context that pregnancy is associated with hypercoagulable changes (Table 24-1).
Table 24-1. Interpretation of Abnormal Coagulation Tests in Pregnancy
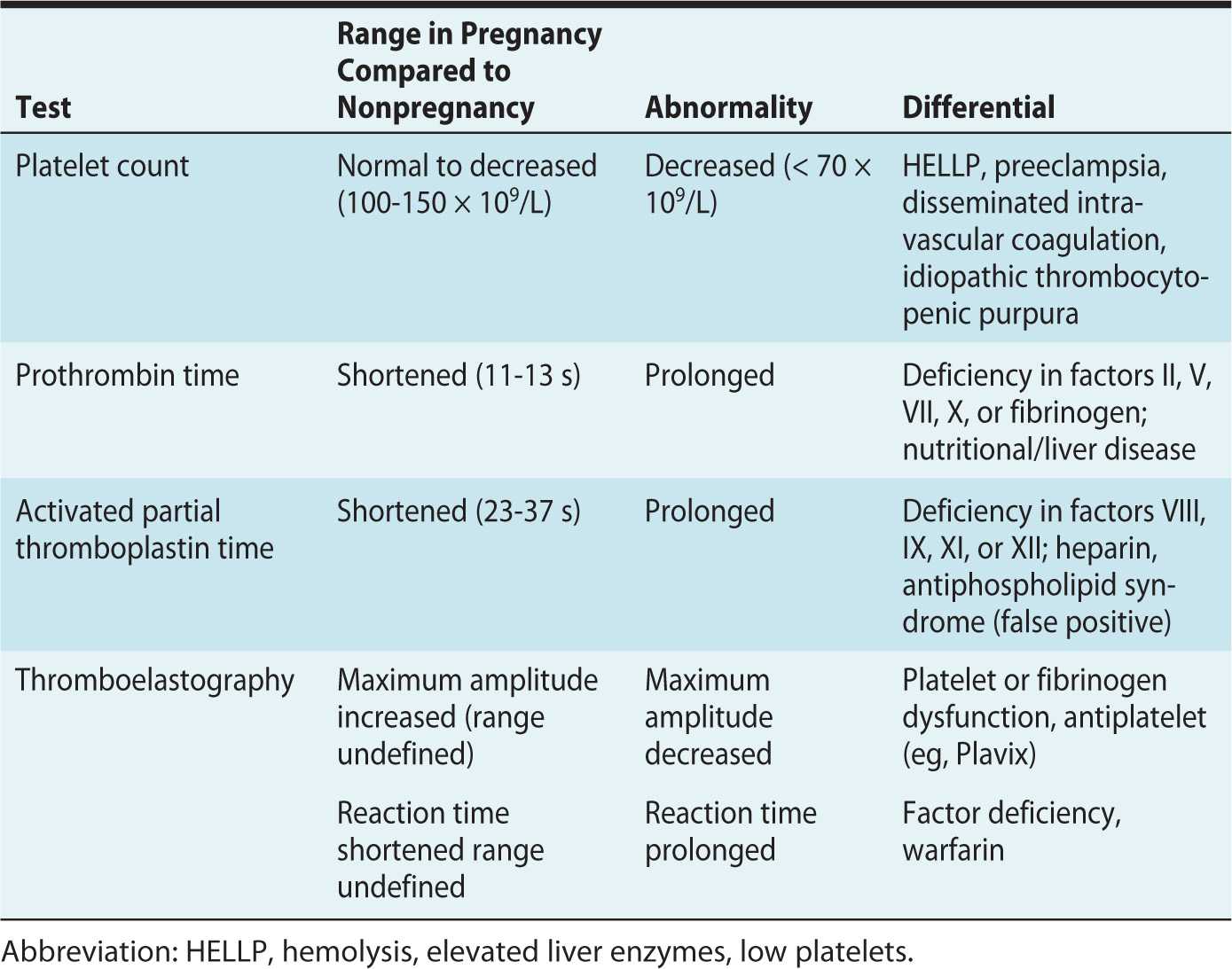
Platelets
Platelet counts decrease in normal pregnancy due to enhanced turnover and hemodilution from plasma volume expansion. The American Society of Anesthesiologists (ASA) does not recommend obtaining a platelet count prior to neuraxial blockade in healthy parturients with no bleeding risk.1,2 However, in some at risk patients, a platelet count of less than 70 × 109/L may be indicative of disease such as hemolysis, elevated liver enzymes, low platelet (HELLP) syndrome, disseminated intravascular coagulation (DIC), or immune thrombocytopenic purpura (ITP).2
Prothrombin Time
The PT and its derived measure, international normalized ratio (INR), evaluate the function of coagulation factors II, V, VII, X, and fibrinogen.2 Prothrombin time (PT) is shortened during pregnancy because of a hormone-related increase in procoagulant factors.2
Activated Partial Thromboplastin Time
Activated partial thromboplastin time (aPTT) evaluates the function of coagulation factors VIII, IX, XI, and XII. The effect of unfractionated heparin can prolong aPTT. A patient with antiphospholipid syndrome and antiphospholipid antibody can have a prolonged aPTT, which represents a false positive test in this prothrombotic subset of patients.2
Thromboelastography
Thromboelastography (TEG) is a bedside test of global coagulation and fibrinolysis. Maximum amplitude is reflective of clot strength and is decreased if platelet function or fibrinogen activity is decreased. The reaction time is prolonged in the setting of a clotting factor deficiency (Table 24-1). TEG is a useful test for dynamic global coagulation, but its widespread use in obstetric centers has been limited by difficulty in standardization of results in the literature.
ANEMIA
Dilutional Anemia of Pregnancy
The physiologic changes of normal pregnancy cause a disproportionate increase in plasma volume (50%) compared to red blood cell mass (30%), resulting in a dilutional anemia in pregnant women.3
EPIDEMIOLOGY
Anemia, defined as a hemoglobin concentration of less than 10 g/dL, occurs in 18% of pregnant women in the developed countries. In contrast, this value is as high as 35% to 75% of pregnant women in developing countries.4 This is likely due to superimposed iron deficiency anemia compounding the physiologic anemia of pregnancy.4
PATHOPHYSIOLOGY
Increased maternal blood volume and cardiac output during gestation are thought to occur primarily as a result of placentally elaborated estrogen and activation of the renin-angiotensin-aldosterone system, which leads to renal sodium reabsorption and water retention.3 The presence of low-resistance circuit, the uteroplacental unit, further augments the need for increased maternal blood volume.
MANAGEMENT AND ANESTHETIC CONSIDERATIONS
The hemodynamic consequences of physiologic anemia and increased plasma volume observed during pregnancy may have an impact on the management of patients with hematologic disease, bleeding risk, or active hemorrhage, as follows:
1. Patients with preexisting pathologic causes of anemia (sickle cell disease, thalassemia) may experience exacerbation of their underlying disease and worsening anemia during pregnancy.
2. In patients at high risk of peripartum bleeding, the detection of underlying anemia may prompt earlier transfusion.
3. The physiologic changes during pregnancy can mask the clinical signs of hypovolemia associated with bleeding, potentially delaying the identification and management of postpartum hemorrhage. A high degree of vigilance for the detection of abnormal bleeding at the time of vaginal or cesarean delivery is important.
Sickle Cell Disease
Sickle cell disease (SCD) is an autosomal recessive disorder involving abnormal hemoglobin (HbS); the most severe form is homozygous. Milder clinical variants occur with coinheritance of other abnormal hemoglobin such as HbC or β-thalassemia.
EPIDEMIOLOGY
SCD is the most common inherited condition worldwide, affecting more than 300,000 children born each year, predominantly in patients of African ancestry and, less frequently, Mediterranean and Middle East populations.5 In the United States, SCD occurs in about 1 in 500 African-American births, and SCD affects about 100,000 Americans. Sickle cell trait occurs among about 1 in 12 African Americans.5
ETIOLOGY/RISK FACTORS
SCD results from inheritance of a mutant version of the β-globin gene (βA) on chromosome 11. The mutant β-allele (βS) codes for variant hemoglobin S; 70% of Americans with SCD are homozygous for βS, whereas others have sickle cell C disease or sickle-thalassemia. The clinical manifestation of SCD is heterogeneous and depends on multiple possible genotypes from hemoglobin alleles and variant regulation of gene expression. SCT is the clinically benign heterozygous carrier state persistent in evolution for its protective advantage against infection by the malarial parasite Plasmodium falciparum.6
PATHOPHYSIOLOGY
SCD manifests clinically as a continuum of chronic hemolytic anemia, recurrent episodic vaso-occlusive crises (VOCs) with severe pain, and progressive end-organ damage.6 The tendency for HbS to sickle when it is in the deoxygenated state exacerbates chronic endothelial dysfunction and vascular inflammation. Acute ischemia, vaso-occlusion, and infarction cause VOCs, and these events occur more frequently in perioperative states of dehydration, acidosis, hypothermia, and increased oxygen demand.6
MANAGEMENT AND ANESTHETIC CONSIDERATIONS
Preconception counseling of women with SCD can identify comorbidities such as hypertension, renal dysfunction, pulmonary hypertension, retinopathy, or iron overload from chronic transfusion requirement, that may be optimized.7 In the setting of chronic anemia, SCD patients can have high-output cardiac failure. An echocardiogram can evaluate baseline cardiac function and delineate the degree of pulmonary hypertension, a known contributor to morbidity and mortality in pregnancy. Iron chelation therapy is contraindicated during pregnancy but may be recommended prior to gestation. Genetic counseling about the risk of inheritance of SCD and screening of partners is important for women with SCD as well as for women with sickle trait. Neonates afflicted with the disease do not develop SCD symptoms until later in life due to the presence of fetal hemoglobin, which contains γ hemoglobin chains in place of β sickle hemoglobin chains.6
Parturients with SCD have a higher incidence of spontaneous abortion, intrauterine growth restriction (IUGR), premature labor, antepartum hospitalization, and postpartum infection.6 The frequency of VOCs increases as pregnancy progresses and reaches a maximum during the third trimester; however, the frequency of VOCs does not correlate with fetal distress, uteroplacental insufficiency, or fetal outcome.
Preanesthesia assessment should focus on the potential for vasculopathy and associated organ dysfunction, with particular attention to transfusion history, stroke history, acute chest syndrome or pulmonary hypertension, VOC frequency, and chronic requirement of pain medication. Principles of anesthetic management include maintaining oxygenation, hydration, normothermia and acid-base neutrality.6
A parturient with SCD may require blood transfusion for symptomatic anemia, acute chest syndrome, or acute stroke.7 Early cross-matching of blood on admission for labor and delivery is important due to the increased risk (8%-50%) of alloimmunization and the presence of atypical antibodies in patients who have received multiple transfusions.6 The presence of atypical antibodies can delay cross-matching of blood products by several hours.
The use of epidural analgesia during labor in women with SCD should be encouraged to prevent increase metabolic demands related to pain.7 The use of epidural analgesia as part of the therapy of a vaso-occlusive pain crisis during labor has also been described.8 Epidural anesthesia for cesarean delivery is preferable to general anesthesia in SCD patients to reduce the risk of hypoxemia, hypotension, and hypothermia, all of which could precipitate a sickling crisis. Adequate lateral tilt position is critical to prevent vena caval compression by the gravid uterus and resulting venous stasis that may contribute to sickling.9
POSTPARTUM CARE
Patients with SCD have a higher potential for infection and risk of VOCs in the puerperium. The reported incidence of complications is 14% to 19% following dilation and curettage and 11% to 17% after cesarean delivery or hysterectomy.10 The use of regional anesthesia may reduce pain-induced respiratory splinting and improve oxygenation in these patients.6 Vigilant management of volume status to avoid dehydration and acid-base imbalance is paramount.
COMMON THROMBOPHILIAS
Thromboembolic disease is the leading cause of maternal mortality in the United States and occurs more frequently in women with underlying thrombophilias.11
EPIDEMIOLOGY
Inherited Thrombophilias
The most common inherited thrombophilias include factor V Leiden heterozygosity, prothrombin gene mutation, plasminogen activator inhibitor (PAI-1) gene mutation homozygosity, and methylenetetrahydrofolate reductase mutation with hyperhomocysteinemia.12 The factor V Leiden mutation or homozygous prothrombin gene mutation is present in 5% to 9% and 2% to 3% of white European populations, respectively, but rare in Asian and African populations.12 Up to 40% and 17% of thromboembolic events in pregnant women have been attributed to factor V Leiden or prothrombin gene mutations, respectively. A homozygous mutation in PAI-1 causing an increase in circulating PAI-1 is relatively common but causes more modestly increased risk of thromboembolism, fetal loss, IUGR, preeclampsia, and preterm delivery compared to other thrombophilias.
Acquired Thrombophilias
The most common cause of acquired thrombophilia during pregnancy is antiphospholipid syndrome (APS), an immune-mediated multisystem disorder strongly associated with poor obstetric outcome.13 The prevalence of APS is approximately 2% to 4%, with more than 50% of affected individuals having a primary form of APS. Generally speaking, 30% of patients with systemic lupus erythematosus (SLE) will develop secondary APS. The most clinically significant antiphospholipid antibodies associated with recurrent pregnancy loss and thromboembolic disease are anticardiolipin antibodies and lupus anticoagulant.13
ETIOLOGY/RISK FACTORS
Inherited or acquired thrombophilias impart increased clotting risk during pregnancy as a result of the background hypercoagulable changes associated with pregnancy—resistance to activated protein C; a decrease in protein S activity; an increase in fibrinogen and factors II, VII, VIII, and X; and an increase in fibrinolysis inhibitors PAI-1 and PAI-2.12 If a woman with a prior history of venous thromboembolic events in the absence of nonrecurrent risk factors (eg, fractures, surgery, prolonged immobilization) is found to have a thrombophilia, then the recurrence risk of venous thromboembolic events is 16% if untreated during pregnancy.14
PATHOPHYSIOLOGY
Thrombophilias are associated with maternal thromboembolic disease during pregnancy, stillbirth, IUGR, abruption, and severe preeclampsia.14 Each of the inherited or acquired thrombophilias affects the balance between prothrombotic and antithrombotic factors in the coagulation pathway. Factor V Leiden mutation impairs the ability of protein C and protein S to inactivate factor Va. Pregnancy-induced reduction in protein S enhances the prothrombotic risk of this mutation.12
MANAGEMENT AND ANESTHETIC CONSIDERATIONS
Women are screened for inherited or acquired thrombophilia by their obstetrician based on the following circumstances: a personal history of venous thromboembolism in the absence of nonrecurrent risk factors, or a first-degree relative with a history of high-risk thrombophilia or venous thromboembolism before the age of 50 years.15 Thrombophilia testing in other situations, such as recurrent fetal loss, IUGR, placental abruption, or history of preeclampsia, is not routinely recommended due to the lack of clinical evidence that anticoagulation therapy prevents occurrence of these complications. Anticoagulation with low-molecular-weight heparin (LMWH) during pregnancy is increasingly recommended for patients with factor V Leiden or prothrombin gene mutations due to emerging evidence that LMWH prophylaxis lowers the risks of fetal loss or thrombotic complications in these women.16
The American Society of Regional Anesthesia and Pain Medicine (ASRA) recently published the third edition of evidence-based guidelines regarding the use of regional anesthesia in patients receiving antithrombotic or thrombolytic therapy (Table 24-2).17
Table 24-2. American Society of Regional Anesthesia and Pain Medicine Guidelines for the Timing of Neuraxial Anesthesia in Patients Receiving Anticoagulant and Antithrombotic Drugs
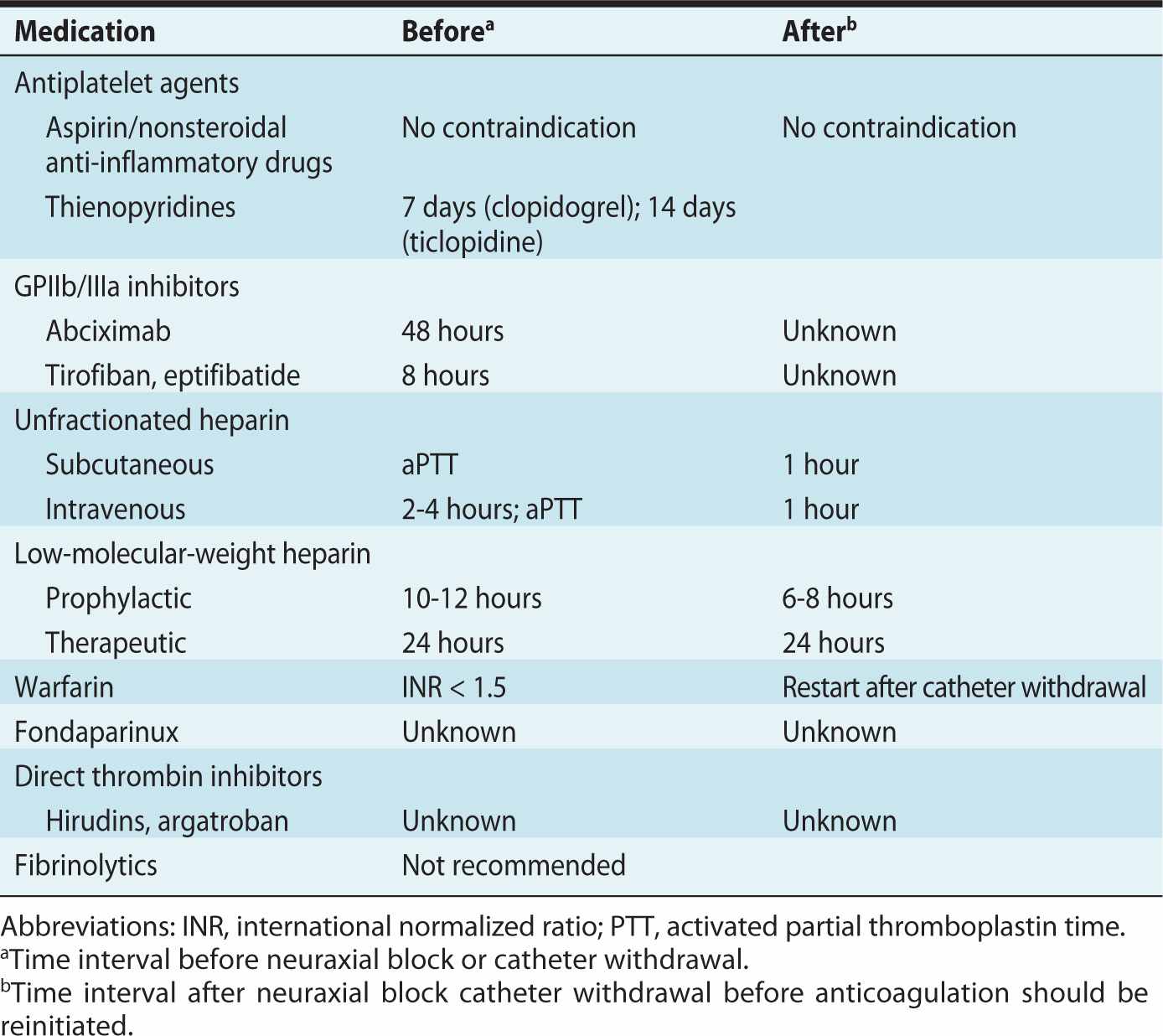
Providing epidural anesthesia for labor or delivery in women treated with prophylactic or therapeutic unfractionated heparin or LMWHs is no longer uncommon, providing the timing is appropriate. Nonetheless, these patients may have a higher risk of developing neuraxial hematoma and this should be included in the discussion of informed consent.2
POSTPARTUM CARE
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








