Fig. 6.1
Schematic of priorities in the management of associated orthopedic injuries in patients with severe head injuries, based on the understanding of the underlying immunological pathophysiology
In TBI patients who have sustained concomitant extracerebral trauma to the musculoskeletal system, a profound systemic inflammatory response is triggered in parallel, involving cytokines/chemokines, complement activation products, the coagulation system, stress hormones, neuronal signaling, and numerous inflammatory cells [28].
The treating surgeon has to be aware of the neuropathology of TBI as well as the systemic inflammatory invents when deciding on the optimal management approach in this challenging patient population, as inappropriate treatment may result in an iatrogenic secondary insult to the brain.
6.3 The “Deadly Duo”: Hypoxia and Hypotension
Episodes of hypoxia and hypotension represent the main independent predictive factors for poor outcome after severe brain injury [8, 29]. In a landmark article published in 1993, Chesnut et al. analyzed the impact of hypotension, as defined as a systolic blood pressure (SBP) <90 mmHg, either during the resuscitation phase (“early”) or in the ICU (“late”), on the outcome of head-injured patients prospectively entered into the Traumatic Coma Data Bank (TCDB) [15]. Early hypotension occurred in 248 of 717 patients (34.6 %) and was associated with a doubling of postinjury mortality from 27 to 55 % [15]. Late hypotension occurred in 156 of 493 patients (31.6 %), of which 39 patients (7.9 %) had combined early and late hypotensive episodes. For 117 patients with an exclusive hypotensive episode occurred in the ICU, 66 % either died or survived in a vegetative state, as defined by a Glasgow Outcome Scale (GOS) score of 1 or 2 points [15]. The authors furthermore determined that mortality is drastically increased in combination with hypotension (SBP <90 mmHg) and hypoxia (PaO2 ≤60 mmHg) [7]. A different study by Elf et al. confirmed the notion, that severe secondary insults occur during the neurointensive care period in more than 35 % of all head-injured patients, including episodes of hypoxia, hypotension, elevated intracranial pressure (ICP) and decreased cerebral perfusion pressure (CPP) [14].
The prevention of hypoxemia and hypotension represents the “key” parameter for avoiding secondary insults to the injured brain and improving outcomes of TBI patients [29, 30]. National guidelines by the Brain Trauma Foundation mandate that blood pressure and oxygenation be monitored in all head-injured patients, and advocate to maintain a systolic blood pressure >90 mmHg and a PaO2 >60 mmHg, respectively [31]. This notion is of particular importance in view of the ongoing debate on the controversial concept of “permissive hypotension” in patients with traumatic hemorrhage from penetrating or blunt torso injuries [32, 33]. The strategy of “permissive hypotension” is mainly based on a landmark article from the 1990s advocating a modified prehospital resuscitation concept for hypotensive patients with penetrating torso injuries, by delaying fluid resuscitation until arrival in the operating room [34]. This proactive concept is certainly intuitive from the perspective that traditional resuscitation with aggressive fluid administration may lead to increased hydrostatic pressure and displacement of blood clots, a dilution of coagulation factors, and an undesirable hypothermia in critically injured patients [35]. However, in light of the vulnerability of the injured brain to secondary insults mediated by hypoxia and hypotension during the early postinjury period, the concept of hypotensive resuscitation, which has seen an unjustified expansion from penetrating to blunt trauma, in absence of high level evidence [32, 36], appears contraindicated for patients with traumatic brain injuries [33, 37].
6.4 Clinical Assessment and Management
Head-injured patients are initially assessed and resuscitated according to the American College of Surgeons’ Advanced Trauma Life Support (ATLS®) protocol [35]. The severity of head injury is diagnosed by the combination of (1) mechanism of trauma, the (2) clinical/neurological status, and (3) imaging by computed tomography (CT) scan. The neurologic status is assessed after stabilization of vital functions [38]. The level of consciousness is rapidly evaluated by the Glasgow Coma Scale (GCS), which grades the severity of TBI as mild (GCS 14/15), moderate (GCS 9–13), and severe (GCS 3–8) [21]. The postresuscitation GCS score is of clinical importance due to the significant correlation with patient outcome [21]. A head CT should be obtained under the following circumstances: (1) altered level of consciousness with GCS <14 (moderate or severe brain injury); (2) abnormal neurological status; (3) differences in pupil size or reactivity; (4) suspected skull fracture; (5) intoxicated patients; and should be repeated whenever the patient’s neurologic status deteriorates [21].
Elevated intracranial pressure (ICP) above 15-20 mmHg has been associated with poor outcomes after severe TBI [39]. Monitoring of ICP by indwelling catheters is recommended under the following conditions [40–43]:
1.
Severe TBI (GCS ≤8) and abnormal admission CT scan
2.
Severe TBI (GCS ≤8) with normal CT scan, but prolonged coma >6 h
3.
Surgical evacuation of intracranial hematomas
4.
Neurological deterioration (GCS ≤8) in patients with initially mild or moderate extent of TBI
5.
Head-injured patients requiring prolonged mechanical ventilation, for example, for management of associated extracranial injuries, unless the initial CT scan is normal
The indications and benefits of emergency craniotomy or decompressive craniectomy are beyond the scope of this chapter, and the reader is deferred to the pertinent peer-reviewed literature [44–46].
Maintenance of an adequate cerebral perfusion pressure (CPP) is recommended above 70–80 mmHg, which is calculated as the mean arterial pressure (MAP) minus ICP [39, 41, 47]. This notion reflects on the imperative not to allow any period of hypotension in head-injured patients, as discussed above [29, 37]. In addition to the outlined dangers of hypoxemia and hypotension, hypercarbia, and hypoglycemia should be strictly avoided or rapidly corrected to minimize the risk of developing secondary brain injuries [14]. Hyperosmolar therapy with mannitol or hypertonic saline is recommended for reduction of cerebral edema and increased ICP, and in patients displaying clinical signs of trans-tentorial herniation, progressive neurological deterioration, or bilaterally dilated and nonreactive pupils [48]. However, the routine use of osmotherapy for management of brain edema represents a topic of heavy debate [49–51]. Similarly, the concept of therapeutic hypothermia for patients with severe head injuries remains controversial [46, 51, 52]. This noninvasive modality of neuroprotection has been investigated for decades in patients with head injuries, cerebrovascular stroke, cardiac arrest, and spinal cord injury [53]. The underlying rationale of moderately lowering the patient’s body temperature is aimed at slowing down the acute inflammatory processes in the injured CNS, and to reduce the extent of traumatic and ischemic tissue injury [54]. Interestingly, the historic euphoria in the 1990s for applying therapeutic hypothermia to patients with severe head injuries [55] was revoked later on in additional validation studies, and the debate on the appropriateness of cooling down the injured brain remains unresolved until present [52, 56]. Despite increased understanding of the pathophysiology of secondary brain injury, the pharmacological “golden bullet” for treating TBI patients and preventing or reducing incidence of secondary cerebral insults has not yet been identified [20]. However, there is unequivocal consensus that the use of steroids is considered obsolete and contraindicated for patients with traumatic brain injuries, since the failure of the large-scale “CRASH” trial was published in 2004 [57, 58].
6.5 Strategies of Fracture Fixation in Head-Injured Patients
Head-injured patients with associated orthopedic injuries represent a vulnerable population due to the high risk of “2nd hit” insults, particularly in presence of femur shaft fractures [17]. The benefits of early definitive fracture stabilization in multiply injured patients are well described and include early unrestricted mobility in conjunction with a decreased “antigenic load” related to stress, pain, and systemic inflammation [13, 59, 60]. Clearly, the question regarding the “optimal” timing and modality of long bone fracture fixation in patients with associated head injuries remains a topic of ongoing discussion and debate [18, 61–64]. Even though the benefits of early femur fracture stabilization have been unequivocally demonstrated in Dr. Bone’s landmark study more than 20 years ago [65], not all multiply injured patients are able to tolerate early definitive fracture fixation due to hemodynamic instability, refractory hypoxemia, or intracranial hypertension [62]. Impressively, experimental studies in sheep showed that femoral reaming and nailing leads to increased ICP levels above 15 mmHg in models of hemorrhagic shock/resuscitation with or without associated traumatic brain injury [19, 66]. A clinical study in 33 blunt trauma patients with TBI revealed that early definitive fracture fixation within 24 h was associated with adverse neurological outcomes and increased mortality, associated with early episodes of hypoxia and hypotension, compared to TBI patients whose orthopedic injuries were stabilized definitively at a later time-points (>24 h) [67]. A larger 10-year study on 61 patients with severe TBI revealed that early femur fracture fixation within <24 h is associated with an increased incidence of secondary brain injury, related to significantly increased rates of hypotension and decreased CPP <70 mmHg [68]. These data were corroborated by a different study analyzing changes in ICP and CPP in 17 patients with severe head injuries undergoing reamed intramedullary nailing of associated femur fractures [69]. The authors showed that the CPP dropped below a minimal threshold of 75 mmHg intraoperatively during the fracture fixation in all patients, with an average decrease in CPP of Δ18 mmHg [69]. The decrease in CPP was attributed to intraoperative episodes of systemic hypotension, and patients with early femoral nailing within 24 h had statistically significant lower CPP values than the rest of the cohort [69].
Overall, there is unequivocal evidence – both from experimental animal studies and from clinical trials in patients with severe TBI – that the early (<24 h) definitive fixation of associated femur shaft fractures in head-injured patients leads to significant adverse effects, including intraoperative episodes of hypotension, increases in ICP and critical decreases in CPP, all of which ultimately constitute preventable “2nd hits” and contribute to secondary brain injury and poor long-term outcomes (Fig. 6.1).
Consequently, alternative strategies to provide early fracture stabilization of long bones, while avoiding the risk of “early total care”, have been proposed, including skeletal traction and “damage control” external fixation [70]. The concept of “damage control” surgery was extended beyond its initial applications in abdominal and thoracic trauma, to the initial management of major fractures in the severely injured, particularly in presence of associated head injuries [62, 71]. The principal is to provide early fracture stabilization by external fixation as a bridge to definitive fracture care once the patient is physiologically stable, and the injured brain less vulnerable to iatrogenic “2nd hit” insults [17]. The delayed conversion from external fixation to intramedullary nailing of femur shaft fractures is considered safe once the ICP has normalized and/or patients are awake, oriented, and fully resuscitated [35]. In other words, the second procedure related intramedullary reaming and nailing of long bone fractures should be performed outside of “priming” window, once the postinjury hyperinflammatory response has subsided (Fig. 6.1). When compared to early total care, the “damage control” approach with delayed conversion to definitive care has been shown to decrease the initial operative time and intraoperative blood loss without increasing the risk of procedure related complications such as infection and nonunion [72, 73].
The risks and benefits of distinct modalities for acute management of femur shaft fractures in head-injured patients, namely (1) skeletal traction [70], (2) “damage control” external fixation [71, 72], and (3) “early total care” by reamed intramedullary nail fixation [69] are depicted in Fig. 6.2.
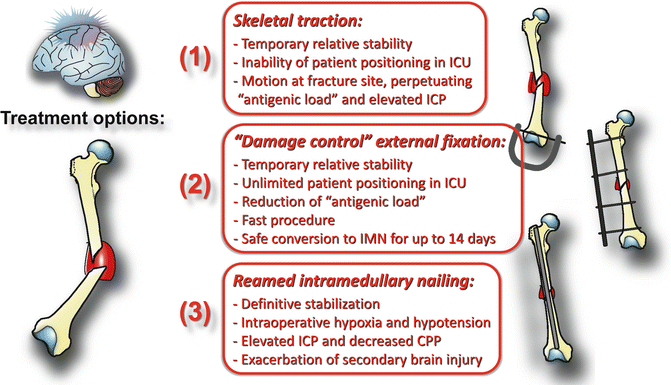

Fig. 6.2
Risks and benefits of distinct management strategies for acute immobilization of femoral shaft fractures in head-injured patients
Conclusion
Head-injured patients with associated long bone fractures represent a very vulnerable patient population [17]. These patients have a high risk of sustaining secondary cerebral insults related to hypotension, increased ICP, and decreased CPP, all of which contribute to increased mortality and adverse neurological outcomes [19, 66–69]. The subspecialties involved in the early management of multiply injured patients with head injuries and associated long bone fractures include ED physicians, trauma surgeons, neurosurgeons, and orthopedic surgeons. They all should be on the same page in terms of understanding the underlying pathophysiology of TBI and the time-dependent vulnerability of the injured brain to iatrogenic “2nd hit” insults [17, 21].
When the patient with combined orthopedic and neurosurgical injuries is evaluated in the emergency department, several questions need to be answered. A rapid neurologic exam must be performed to assess the severity of brain injury. A noncontrast craniocerebral CT scan is obtained as the first-line adjunctive diagnostic work-up in stable patients. An ICP monitor (either fiberoptic or ventricular) may be placed in the ED if the patient is too hemodynamically unstable to justify a trip to the CT scanner.
Any patient with a suspected brain injury who needs to be taken to the operating room and will be unable to undergo follow up neurologic examination needs to have ICP monitoring. The exact ICP threshold of when not to proceed to the operating room is unknown, though sustained pressures beyond 15–20 mmHg should be an indication to proceed to the ICU for resuscitation. Any patient with a progressively worsening neurological exam is also at high risk as is the patient with unexplained changes in ICP. Hypoxia and hypotension significantly increase mortality in the patient with brain injury.
Despite recent advances from basic research and clinical studies [74], the current literature remains conflicting in terms of identifying a clear-cut management strategy for timing and modality of fracture fixation in severely head-injured patients [17, 18, 61, 64, 67, 68]. This notion emphasizes the pressing need for well-designed prospective controlled multicenter trails aimed at comparing the standard treatment strategies for initial management of long bone fractures in patients with severe head injuries (Fig. 6.2 ).
Until higher level evidence-based recommendations are available, the clinical approach for the management of this vulnerable cohort of patients must be based on the basic principle of “do not further harm” by applying simple measures of “damage control” – when in doubt – which respect the underlying pathophysiology of traumatic brain injury and the hyperinflammatory response of the combination of multiple critical injuries [13]. We recommend the following specific management strategy for associated orthopedic injuries in head-injured patients, based on a combination of empiric experience and review of the available pertinent literature in the field:
1.
“Damage control orthopedics” by spanning external fixation in all patients with severe TBI (GCS ≤8, intracranial pathology on CT scan, including cerebral edema, midline shift, sub-/epidural bleeding, or open head injuries).
2.
Optional “damage control orthopedics” in all patients with moderate TBI (GCS 9–13), or patients with GCS of 14/15 with “minor” intracranial pathology on CT scan (e.g., traumatic subarachnoid hemorrhage that warrants observation only). Concomitant neurosurgical procedures may be performed at the same time as DCO, for example, an emergency craniotomy.
3.
No additional operations (2nd hit) in patients with refractory intracranial hypertension or unexplained deterioration in neurologic exam.
4.
Conversion from external to internal fixation in TBI patients who recovered from a comatose state and are awake and alert (GCS 13–15), or comatose patients with a stable ICP (<20 mmHg) and CPP in a normal range (>80 mmHg) for more than 48 h.
5.
“Early total care” for long bone fractures all patients with mild TBI (GCS 14/15) and normal initial craniocerebral CT scan.
6.




Temporary skeletal traction as a valid adjunct for patients “in extremis”, that is, in severe protracted traumatic-hemorrhagic shock and coagulopathy, who are unsafe to be taken to the operating room until adequately resuscitated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







