• Pregnancy with multiple fetuses
• Fetal macrosomia
• Fetal malpresentation
• Prior uterine incision or myomectomy
• Prolonged labor
• Prolonged third stage (>20 min)
• Previous postpartum hemorrhage
• Placenta previa
• Maternal obesity (BMI > 40)
• Inherited coagulopathy
Etiologies of Primary Postpartum Hemorrhage
Uterine atony, the failure of the uterus to contract following labor, is the most common cause of primary PPH [7, 24]. Risk factors for uterine atony include conditions causing overdistention of the uterus such as multiple birth pregnancy and fetal macrosomia, prolonged labor, and deep anesthetic use [25–27]. Maternal obesity (body mass index 40 or higher) increases the risk of atonic uterine hemorrhage [28]. Although breast-feeding may have a uterotonic effect due to endogenous oxytocin, this does not necessarily prevent PPH due to uterine atony [24].
An atonic uterus is “boggy” from the failure of myometrial fibers to contract. It is palpable on bimanual exam as softer than a typical, contracted post-delivery uterus. Initial management consists of bimanual uterine massage, which stimulates myometrial fibers to contract. Bimanual massage involves forceful compression of the uterus, with one hand providing external pressure on the lower abdomen and the other hand providing intravaginal pressure on the cervix [26] (Fig. 8.2). Bimanual compression is more effective when performed by a two-person team. One provider maintains external pressure to the uterine fundus, while a second provider places pressure on the lower uterine segment. This technique allows for sustained duration of compression, which may be required to achieve effective uterine contraction [29].

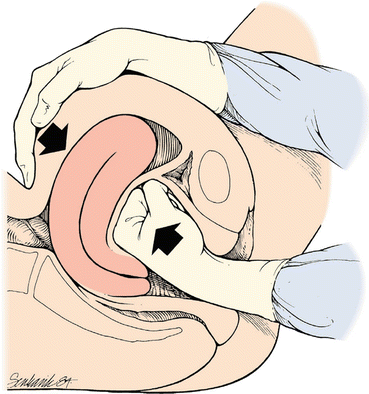

Fig. 8.1
The above case, a patient presenting to the ED with severe secondary PPH, illustrates some of the unique management challenges requiring coordinated efforts across departments in order to optimize care

Fig. 8.2
Bimanual uterine massage. Francois KE, Foley MR. Antepartum and postpartum hemorrhage. Obstetrics: Normal and Problem Pregnancies. Ed. Steven G. Gabbe. Philadelphia: Elsevier, 2016. 407. Print. With permission
Traumatic lacerations to the vagina and cervix during delivery are another cause of PPH. Direct visualization of the vaginal walls and cervix is required for diagnosis and repair. Although ongoing hemorrhage will likely obscure a clear view, all possible efforts should be made for direct inspection after uterine atony has been excluded and treated by examination and bimanual compression [7].
Placenta that is partially or completely retained can cause hemorrhage shortly after delivery. Examination of the placenta after delivery is necessary to ensure it is complete, without any missing segments that may remain in the uterus. Retained placenta must be removed for bleeding to stop. Manual removal is achieved by sweeping the uterus digitally or with surgical instruments; mechanical evacuation of retained placenta is more effective than pharmacologic maneuvers [30, 31].
Abnormal placental implantation can also contribute to PPH [9, 32]. In the case of placenta accreta, the placenta invades the myometrium and incompletely separates at birth, leading to open sinuses, which predispose to severe hemorrhage [32]. Placenta accreta is subclassified into placenta increta or placenta percreta, based on involvement of placenta into or through the myometrium [27]. When retained products are due to placenta accreta, PPH can be especially difficult to control and hysterectomy is often necessary. Risk factors for placenta accreta include prior placenta previa and prior invasive gynecologic and obstetric procedures including uterine incisions, endometrial ablation, and uterine artery embolization [22].
Uterine inversion is a life-threatening but rare obstetric complication that can lead to postpartum hemorrhage. It is identified by visualization or palpation of the uterine fundus in the introitus or vaginal vault and by the inability to palpate the uterine fundus in the abdomen. Associated shock syndrome is due to blood loss, with some component of parasympathetic response to excessive traction on the malpositioned uterus [32–34]. The severe degree of shock may appear out of proportion to visualized blood loss. Incomplete inversion can occur with a more occult presentation, in which the fundus inverts but remains within the body of the uterus [33]. Ultrasound is helpful in confirming the diagnosis if it is unclear based on physical exam alone. Ultrasound may show an “inside out” or “upside down” sign, with the fundus displaced toward the vagina or in the uterine body [35]. Although often attributed to adherent placenta or excessive cord traction, the cause of uterine inversion is often unknown. Table 8.2 summarizes the commonly reported etiologies of primary PPH.
Table 8.2
Etiologies of postpartum hemorrhage
Primary hemorrhage: | Secondary hemorrhage: |
• Uterine atony • Traumatic lacerations • Retained placenta • Abnormal placentation • Placenta accrete • Coagulopathy • DIC • TTP • ITP • HELLP • Von Willebrand disease • Thrombocytopenia • Hemophilia carrier • Uterine inversion | • Uterine atony • Subinvolution of placental site • Retained products of conception • Retained placenta • Endometritis • Coagulopathy • DIC • Uterine artery pseudoaneurysm |
Etiologies of Secondary Postpartum Hemorrhage
Secondary postpartum hemorrhage occurs in approximately 1% of pregnancies, and many identifiable causes overlap with those implicated in primary PPH. However, in one study spanning 9 years, no cause was determined in 16.7% of cases of secondary PPH, and when determined, the diagnosis was most often based on histopathologic findings. As with primary PPH, uterine atony, retained products of conception, subinvolution of the placental site, and coagulopathies are the etiologies most often identified [36] (Table 8.2).
Retained products of conception (POC) commonly present as a cause of secondary PPH. Persistence of the trophoblastic villi and increased vascularity to retained POC are sources of hemorrhage. Retained placenta can also lead to subinvolution of the placental site and uterine atony, leading to multifaceted causes for hemorrhage. Ultrasound imaging may show a thickened endometrium or intrauterine mass with vascular color flow on Doppler [37].
Placental site subinvolution is the failure of uteroplacental vessels to close after delivery. Normally after delivery, placental vessels involute to constrict the dilated uteroplacental vessels caused by normal pregnancy physiology. In the case of subinvolution, arteries remain dilated when they should be closing, which can lead to massive blood loss postpartum [38]. Subinvolution was found as the cause of 13.3% of cases of secondary postpartum hemorrhage in one report. It is often associated with retained products of conception [36]. A diagnosis can be suggested by ultrasound showing increased myometrial vascularity and low resistance flow, which can appear similar to retained POC or arteriovenous malformation [39].
Endometritis, an infection of the endometrium after delivery by a combination of aerobes and anaerobes, is another causative factor in secondary PPH [36]. Characterized by fever, pelvic pain, and uterine tenderness with or without purulent lochia, endometritis can rapidly progress to toxic shock syndrome, sepsis, or necrotizing myometritis. Postpartum infections also predispose the patient to hemorrhage due to acquired coagulopathies. Release of cytokines associated with severe infection leads to activation of the fibrinolytic system and the coagulation cascade, which can quickly progress to disseminated intravascular coagulation (DIC). Specifically, a few cases have been reported of endometritis due to Clostridium bacteria, which release a hemorrhagic toxin leading to toxic shock syndrome [40]. Hemodynamic instability can occur with hemorrhagic and septic shock occurring simultaneously. Treatment should include broad-spectrum antibiotic coverage of anaerobic and gram-negative bacteria [41].
Uterine artery pseudoaneurysm, a rare cause of secondary PPH, is a collection of blood that communicates with arterial blood flow. It is usually associated with cesarean delivery, as there is potential for trauma to the uterine artery. Pseudoaneurysm occurs when a hematoma forms around leakage from the uterine artery and a communication persists. Hemorrhage can be intra-abdominal or vaginal depending on the location of the hematoma [42]. Alternatively, the pseudoaneurysm can rupture, leading to sudden and potentially massive blood loss [43].
Hematologic Considerations in Postpartum Hemorrhage
Physiologic adaptations in pregnancy prepare the patient for blood loss during the childbirth process. In addition to increased blood volume, serum clotting factors increase in pregnancy, including fibrinogen levels, von Willebrand factor, and levels of factors VII, VIII, IX, and X [25, 44]. Both inherited and acquired coagulation defects will predispose the patient to PPH.
Patients with inherited bleeding disorders require special care to prevent PPH [45]. Von Willebrand disease (vWD), the most common hereditary bleeding disorder, is present in approximately 1% of the population and may be mild or severe. Women with vWD may report a history of excessive menstrual bleeding [46]. Patients who are hemophilia carriers will have variable deficiencies in clotting factors; patients with the lowest concentrations of factor levels in the third trimester have higher incidence of PPH. Patients with known bleeding disorders are recommended to receive factor treatment prior to delivery to prevent PPH [45, 47]. In cases of PPH in patients with hereditary bleeding disorders, management involves replacement of deficient clotting factors [47].
Acquired bleeding disorders in the postpartum period include disseminated intravascular coagulation (DIC), quantitative platelet disorders including immune thrombocytopenic purpura (ITP), thrombotic thrombocytopenic purpura (TTP), and the HELLP syndrome of hemolysis, elevated liver enzymes, and low platelets [48].
DIC is a major concern in postpartum hemorrhage. DIC is a life-threatening consumptive coagulopathy that leads to microvascular thrombosis and may lead to organ failure. Exsanguinating PPH in the context of DIC is a complex syndrome involving consumption of coagulation factors and inability to stop bleeding after placental separation. Amniotic fluid contains procoagulants including a direct factor X activator as well as complement. An excess of tissue factor (TF) is released during detachment of the placenta from the uterine wall after delivery [49]. Placental tissue and syncytiotrophoblasts express higher levels of TF than other endothelial cells, and levels of TF are increased during the third trimester in a physiologic effort to prevent fibrinolysis. If TF is released into maternal circulation, as with placental abruption, amniotic fluid embolism, and placenta accreta, systemic activation rather than local activation of coagulation is initiated, leading to potentially widespread coagulation and depletion of platelets and fibrinogen [49]. Activation of the fibrinolytic system is followed by activation of the coagulation cascade [50]. Sepsis and infection can also lead to DIC through cytokine release [51].
Although characteristic laboratory features often define DIC, it is largely a clinical diagnosis. Identification of coagulopathy with labs including PT, PTT, fibrinogen, fibrin split products, D-dimer, and platelet levels may not be diagnostic in the patient in the immediate postpartum period. Typically, low fibrinogen levels are diagnostic of DIC for nonpregnant patients. However, fibrinogen is an acute-phase reactant and is unlikely to be low, even in pregnant or postpartum women with DIC, unless massive postpartum hemorrhage has occurred. It is important to trend fibrinogen levels in this setting, paying close attention to downward trends. D-dimer levels, which are normally elevated during pregnancy, are likely of little clinical value in bleeding pregnant or postpartum patients [49].
Emergency Department Management of Postpartum Hemorrhage
First-Line Treatments
Nearly all patients presenting to the ED with PPH will require simultaneous diagnostic evaluation and stabilization measures. Involvement of consultants from obstetrics and interventional radiology should be requested early in the course of care of the patient with PPH. The initial history and physical exam may provide insight into causes based on complications during pregnancy, history of bleeding, and cesarean versus vaginal delivery. Physical exam may reveal a boggy/atonic uterus or obvious traumatic laceration. Bimanual uterine compression during the initial examination may be therapeutic for controlling PPH. Initial laboratory tests include complete blood count, coagulation factors, and type and screen. Ultrasonography may reveal evidence for retained products of conception. If uterine inversion is detected, management involves placing the fundus back into the correct position. Gentle pressure on the uterine fundus with the palm of the hand as if holding a tennis ball will help correct positioning, but may require uterine relaxants such as magnesium and nitroglycerin to allow repositioning to occur [33, 52]. Pain should also be addressed. When PPH is severe, as indicated by observed volume of blood loss, an elevated shock index, or other markers such as elevated serum lactate, blood transfusion should be considered prior to a measurable fall in the patient’s hemoglobin and hematocrit [19]. Emergency transfusion of uncrossmatched O negative blood or type-specific blood should be initiated as soon as severe PPH and hypovolemic shock are recognized [53].
If the uterus is palpably firm, or manual compression is not slowing bleeding, careful inspection of vaginal walls, cervix, and perineum should be completed for evaluation of tears or lacerations.
Pharmacologic Therapies
Several pharmaceuticals have the ability to stimulate the smooth muscle of the uterus. A familiarity with these agents, which are seldom used in the ED, can facilitate patient care during urgent situations involving PPH.
Oxytocin. Oxytocin, a synthetic formulation of the nonpeptide pituitary hormone, has stimulant effects on the smooth muscle of the uterus. Oxytocin stimulates uterine contractions by increasing intracellular calcium [54]. Oxytocin may be given prophylactically at the third stage of labor, and this has been shown to reduce PPH [55]. The initial dose is 10–40 units in 1000 ml crystalloid solution given as a continuous IV infusion [7] or up to 10 units intramuscular (IM). Rapid, undiluted IV administration should be avoided as this can cause hypotension. Oxytocin is the drug of choice for prophylaxis and treatment of PPH [56].
Methylergonovine. Midwives have used ergot alkaloids for centuries before being acknowledged by the medical profession in the mid-1800s. Ergot alkaloids directly stimulate uterine contraction. Methylergonovine is a semisynthetic ergot alkaloid that acts directly on the smooth muscle of the uterus and increases uterine contractions. The dose is 0.2 mg intramuscularly (IM) every 2–4 h [7], and onset of action is within minutes. Side effects include nausea and vomiting [55]. Methylergonovine should be avoided in women with a history of preeclampsia or coronary artery disease, as it can cause hypertension and vasospasm.
Prostaglandin analogs. Misoprostol is a prostaglandin E1 analog that induces contraction of the smooth muscle fibers of the myometrium. It can aid in contraction of the uterus to slow bleeding due to uterine atony [57]. It is used adjunctively in the management of retained placenta, but with inconclusive evidence as to its benefit over manual removal [30]. Misoprostol may be administered orally, buccally, sublingually, vaginally, or rectally with onset of action between 8 and 11 min when given via the oral or buccal route [58–60]. The recommended dose is 800–1000 mcg rectally [7]. When administered for postpartum hemorrhage, higher incidences of pyrexia have been described [61].
In cases of severe PPH, oxytocin, methylergonovine, and misoprostol may all be administered. Efficacies of methylergonovine and misoprostol each as single agents have not been found to be as efficacious as oxytocin alone [55, 62]. Misoprostol in conjunction with oxytocin to reduce PPH is associated with increased side effects of shivering and fever as compared to single therapy [62].
Desmopressin (DDAVP) is indicated in the management of PPH in patients with vWD and hemophilia carrier states; factor supplementation is indicated in patients with known deficiencies [47]. Factor VIIa administration has utility in patients with PPH complicated by coagulopathy, and antifibrinolytic therapy is an additional option for the prevention and control of PPH in patients with bleeding disorders [47, 63, 64]. The World Maternal Antifibrinolytic Trial (WOMAN Trial) of the use of tranexamic acid (TXA) has shown potential benefit for prevention of PPH in low-risk patients, but mixed reviews challenge its efficacy in the adjunctive treatment of active PPH [6, 64–66].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






