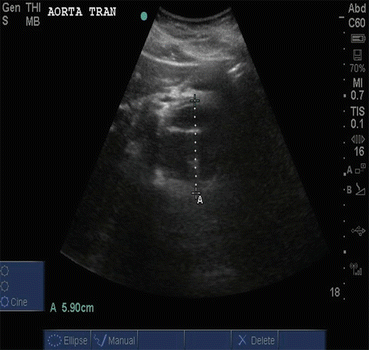
Fig. 9.1
Ultrasound image of decreased blood flow through right femoral vein DVT in transverse view. Reprinted with permission of the MedStar Georgetown University Hospital & MedStar Washington Hospital Center Emergency Ultrasound Group
Pulmonary Embolism
The diagnosis of PE is difficult in the pregnant patient as the clinical signs and symptoms of PE (dyspnea, tachycardia, lower extremity edema) occur in normal pregnancy. Clinical decision rules have barely been studied in pregnant patients, and none have been validated in the pregnant population. Standard diagnostic tools such as D-dimer lose accuracy as levels increase physiologically during normal pregnancy. As such, physicians tend to test for PE at lower thresholds in pregnant patients and rely heavily on diagnostic imaging studies. Indeed, the incidence of PE is 5% or less in most studies of pregnant patients with suspected PE, compared to 20–25% in nonpregnant patients [7]. Several diagnostic algorithms have been proposed, but the optimal strategy to diagnose PE in pregnancy remains under debate.
The D-dimer represents a breakdown product of cross-linked fibrin and is a useful screening tool for DVT/PE in the nonpregnant population. In pregnancy, however, the D-dimer level increases steadily with each trimester, making the test less useful to rule out VTE. Multiple studies have evaluated trimester-adjusted thresholds for normal D-dimer levels in pregnancy, but results are inconsistent [8–12]. Even if “normal range” pregnancy values are firmly established, the threshold to safely exclude VTE would need to be identified. Some authors and guidelines state that a negative D-dimer is still reliably negative in a pregnant patient with low pretest probability [13, 14]. Other guidelines are contradictory and conclude that the D-dimer cannot currently be used to exclude suspected PE in pregnancy [15].
The diagnostic work-up of patients with suspected PE often begins assessment of pretest probability using a validated clinical decision rule. The modified Wells score (MWS) is a validated clinical tool (in nonpregnant patients) used to risk stratify patients in whom there is clinical concern for PE (Table 9.1). It is often used in conjunction with a D-dimer to guide the work-up.
Table 9.1
Modified Wells scorea—prediction rule for diagnosing PE
Clinical symptoms of DVT | 3 |
Other diagnosis less likely than PE | 3 |
Pulse >100 | 1.5 |
Immobilization ≥3 days or surgery in previous 4 weeks | 1.5 |
Previous PE or DVT | 1.5 |
Hemoptysis | 1 |
Malignancy | 1 |
Low probability | 0–1 |
Intermediate probability | 2–6 |
High probability | >6 |
Several studies have evaluated the MWS in pregnancy. A retrospective review by O’Connor et al. [17] applied the MWS to 81 pregnant and 22 postpartum patients referred for CTPA over a 5-year period. The authors found that an MWS of 6 or greater was 100% sensitive and 90% specific with a positive predictive value of 36% for PE on CTPA. This study is small and retrospective and lacked follow-up data, but shows a promising application of the MWS in pregnant patients.
A second study by Parilla et al. [12] evaluated trimester-specific D-dimer levels combined with the MWS as useful risk stratification tools in pregnant women in a prospective and retrospective cohort study. While the number of patients included in the study was low, the results were promising. Using both trimester-specific D-dimers and an MWS was 100% sensitive and 81.4% specific in detecting PE if either of the results were abnormal. In this study, all of the patients with PE would have received a CTPA, and the total number of CTPAs performed would have been decreased by 52.5%.
Currently, imaging tests are the mainstay of diagnostic management of suspected PE in pregnancy. When choosing an imaging modality, risks to the fetus and mother must be considered. The two most commonly used studies for suspected PE are CTPA and V/Q scan. VQ scan confers a higher dose of radiation to the fetus, while CTPA delivers more radiation to the mother, particularly to breast tissue. Breast tissue of pregnant women has a high rate of cell turnover and thus more susceptible to ionizing radiation. However, CTPA can identify alternate pathology. Both forms of imaging confer radiation doses much lower than the exposure associated with fetal harm (Table 9.2).
Table 9.2
VQ scan vs. CTPA for evaluation of pulmonary embolism
VQ scan | CTPA | |
|---|---|---|
Fetal radiation exposurea | 0.37 mGy dose Radiation exposure greater to fetus | 0.01–0.66 mGy Radiation exposure greater to mother, especially maternal breast tissue |
Iodinated contrast exposure | N/A | Contrast exposure with possible induction of neonatal hypothyroidism; FDA classifies iodinated contrast as “B” level recommendation for safety |
Diagnostic value | Rate of nondiagnostic study is between 7 and 21% | Can identify pathology other than PE |
Multiple diagnostic algorithms for suspected PE in pregnancy have been proposed. The American Thoracic Society and the Society of Thoracic Radiology published one such clinical practice guideline in 2011 [15]. A multidisciplinary panel including members of ACOG was convened to develop evidence-based recommendations. Overall, the panel found that there was “limited amount of direct evidence pertaining to diagnostic test accuracy and patient-important outcomes in the pregnant population.” Nonetheless, recommendations were made and are summarized in Fig. 9.2. If the patient has leg symptoms (swelling, calf pain, or asymmetry), bilateral lower extremity ultrasounds to evaluate for DVT should be the starting point. If a DVT is found, begin treatment without further work-up. If there are no leg symptoms, the first step in the evaluation of a PE should be a chest X-ray (CXR) as it may show other etiologies that explain the patient’s symptoms (e.g., pneumonia, pulmonary edema). In the pregnant patient with a normal CXR and no history of pulmonary disease (such as asthma), V/Q scan is recommended. If the patient has an abnormal CXR or a history of pulmonary disease, CTPA is recommended.
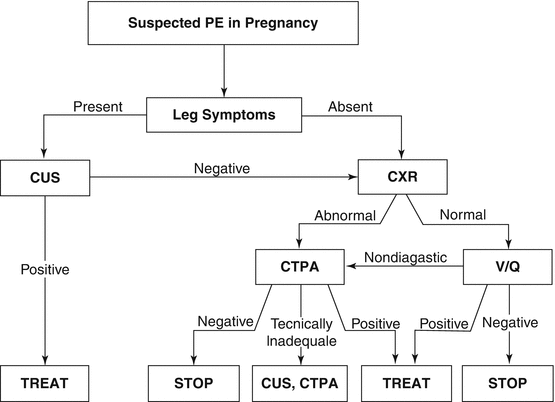

Fig. 9.2
Diagnostic algorithm for suspected PE in pregnancy. (Reprinted with permission of the American Thoracic Society. Copyright © 2016 American Thoracic Society. Leung AN, et al. [15]. The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society. CUS compression ultrasonography, CXR chest X-ray, CTPA computed tomographic pulmonary angiogram, V/Q ventilation perfusion)
Other algorithms have been proposed and clinical practice varies widely. Several authors recommend obtaining bilateral lower extremity ultrasounds as a first step when either DVT or PE is suspected [18, 19]. If chest imaging is necessary, the authors believe both CTPA and V/Q scans are equally justifiable for the pregnant patient. The authors prefer CTPA as it may identify alternate pathology. Additionally, there is a risk that the V/Q scan may be indeterminate, requiring that a CTPA be performed as well (Fig. 9.3).
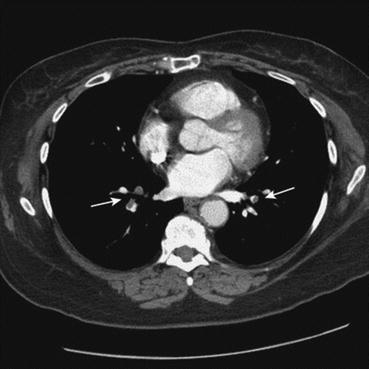

Fig. 9.3
CTPA revealing intraluminal filling defects (arrows) in the pulmonary vascular tree. From Sibai BM, eds. Management of Acute Obstetric Emergencies. Philadelphia: Saunders, 2011; With permission
Once the diagnosis of PE/DVT is made in a pregnant patient, treatment with either low molecular weight heparin (LMWH) or unfractionated heparin (UFH) is indicated. Neither drug crosses the placenta and both are considered safe in pregnancy. UFH is recommended instead of LMWH in patients with GFR less than 30 mL/min or in situations where delivery, surgery, or thrombolysis is imminent. Thrombolytic therapy should be considered for pregnant women with life- or limb-threatening VTE [20]. Warfarin is contraindicated in pregnancy because it is associated with teratogenic fetal effects. To the authors’ knowledge, there is no controlled human data regarding novel anticoagulants and pregnancy.
Disposition of pregnant patients with newly diagnosed VTE is similar to nonpregnant patients, but decisions should be made in close consultation with the patient’s obstetrician. In general, patients who are clinically stable with no major risk factors for bleeding and easy access to medical care may be treated as outpatients. Hospitalization is indicated in patients who are hemodynamically unstable and have extensive VTE or maternal comorbidities that increase their risk of major bleeding or in patients that require treatment with UFH.
Aortic Dissection
A rare but devastating cardiovascular emergency of pregnancy is a major vessel arterial dissection. While an overall uncommon event, the majority of aortic dissections in women of childbearing age occur during pregnancy. Indeed, for women younger than 40, pregnancy is associated with a markedly increased risk of aortic dissection (odds ratio of 25) [21]. The role of the emergency physician includes early recognition, resuscitation, early activation of institutional massive transfusion protocols, immediate surgical and obstetrical consultation, and overall coordination of care.
The well-described physiologic and hemodynamic changes of pregnancy in concert with accompanying hormonal changes create significant new stresses on the circulatory systems. The gravid uterus compresses the abdominal aorta and iliac arteries, leading to increased vascular resistance at the point of compression and increased stress on the intimal layers of the more proximal aorta. It is suspected that these stresses, in combination with increased effective circulating volume, heart rate, cardiac output, and other hemodynamic changes of pregnancy, create new strains on the vasculature of even previously healthy pregnant patients. The role of elevated progesterone and, more importantly, estrogen augments these hemodynamic stresses. As early as the first trimester, maternal arterial vessels begin to demonstrate increased compliance [22]. Postmortem histopathologic examination from fatal aortic dissections in pregnant patients demonstrates severe degeneration of elastin fibers and other markers of “arterial degeneration” [23]. Patients with known connective tissue disorders or conditions (Ehlers-Danlos, Marfan’s, and Turner’s syndromes) are also at increased risk for acute aortic dissection.
A recent nationwide study from the Netherlands [24] examined vascular dissections causing death in 23 pregnant patients (incidence 0.74 per 100,000 live births). The authors observed the most frequent location of dissection to be the aorta, with coronary and splenic artery dissections less common. The clinical presentation for a thoracic aortic dissection was variable, but the majority of patients had classic complaints of severe, sharp chest pain described as “ripping” and/or “tearing” and radiating to the back, sometimes accompanied by nausea and vomiting. However, making the diagnosis is challenging. Undoubtedly, the clinical presentation of dissections often significantly overlaps with other cardiovascular emergencies in the pregnant patient such as PE, acute coronary disease, amniotic fluid embolism, or preeclampsia. As described previously in the VTE section, D-dimer is not a useful screening tool as levels rise steadily throughout normal pregnancy.
To definitively diagnose aortic dissection, imaging is required. Similar to nonpregnant patients, CT or transesophageal electrocardiography (TEE) may be used. TEE is ideal in pregnancy as it confers no ionizing radiation and can be performed in the ED, but is not always available. CT does confer ionizing radiation and requires intravenous contrast; however, these risks must be weighed against the risks of missing a potentially lethal vascular catastrophe. Importantly, a necessary diagnostic test should not be withheld from a pregnant patient out of concern for possible risk to the fetus. Bedside ultrasonography may also be useful. Findings such as intimal flaps within the abdominal aorta, grossly dilated vascular size, a false lumen, or hemopericardium warrant emergent angiography or other more immediate action depending on the patient’s hemodynamic status (Fig. 9.4).