TOPICS
THROMBOEMBOLIC DISORDERS
Epidemiology
Pregnancy imparts a four fold to five fold increased risk of thromboembolism when compared to the nonpregnant state.1 This risk rises to a 20-fold increase during the postpartum period and does not return to nonpregnant levels until approximately 6 weeks’ postpartum.1,2 The majority of thromboembolic events in pregnancy are venous in origin. The incidence of venous thromboembolism (VTE) in pregnant women is estimated to be 5 to 12 events per 10,000 pregnancies, evenly distributed between the time period from conception to delivery.1 The mortality from pregnancy related VTE is 1.1 deaths per 100,000, an estimate of about 10% of all maternal deaths.3
Types of Venous Thromboembolism
Venous thromboembolism in pregnancy is commonly manifested as pulmonary embolism or as deep venous thrombosis (DVT). DVT accounts for 80% of thromboembolic cases, while pulmonary embolism is responsible for the remaining 20%.4
PULMONARY EMBOLISM
Pulmonary embolism (PE) is the leading cause of direct maternal deaths in developed countries, and it accounts for 20% of pregnancy-related deaths.3 The incidence of PE is 0.01% to 0.05% of all pregnancies, and the risk is greater in the postpartum period, with 43% to 60% of pregnancy-related episodes occurring 4 to 6 weeks postpartum. PE after cesarean delivery is higher than after vaginal delivery by a factor of 2.5 to 20, and the incidence of fatal PE is higher by a factor of 10.2
DEEP VENOUS THROMBOSIS
The incidence of DVT is 0.02% to 0.36% of all pregnancies. A meta-analysis showed that two-thirds of cases of DVT occur antepartum and are equally distributed across trimesters.5 Pregnancy-associated DVT is left sided in more than 85% of cases. The mechanism for this predilection for the left leg is probably related to compression of the left iliac vein by the right iliac artery and the gravid uterus (Figure 19-1).1
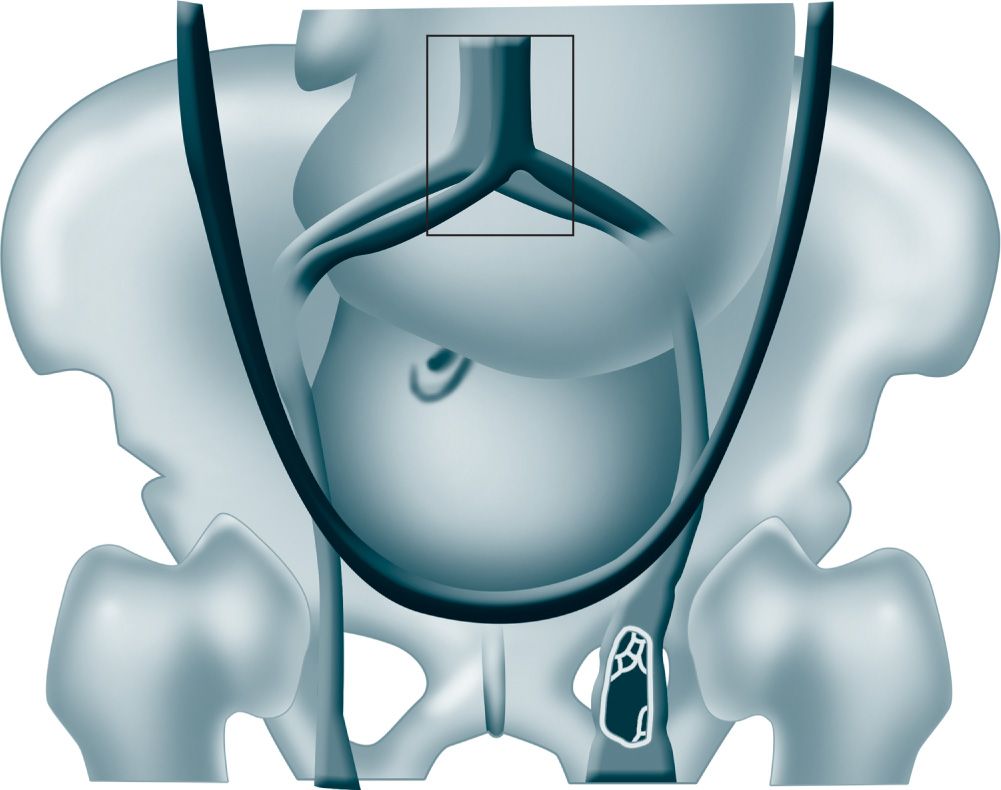
Figure 19-1. Mechanism for predilection for left leg deep venous thrombosis likely related to compression of the left iliac vein by the right iliac artery and the gravid uterus. (From Bourjeily G, Paidas M, Khalil H.1, with permission.)
PELVIC VEIN THROMBOSIS
Isolated pelvic vein thrombosis (PVT) is more common in pregnancy. According to a multicenter prospective registry, 11% (6 of 53) of pregnant or postpartum women with DVT had isolated PVT compared with 1% (17 of 5451) of nonpregnant patients.1 Ovarian vein thrombosis, a form of septic PVT, may complicate less than 0.05% of vaginal deliveries and up to 1% to 2% of cesarean deliveries. In 90% of cases, PVT occurs within 10 days’ postpartum but can occur up to 10 weeks’ postpartum. Symptoms include fever unresponsive to antibiotics (80%), pelvic pain (66%), and a palpable abdominal mass (46%).6
SUPERFICIAL VEIN THROMBOSIS
Superficial vein thrombosis (SVT) involving the lower extremity is occasionally noted during pregnancy. A retrospective study estimated the incidence of SVT during the peripartum period to be 47 out of 72,000 deliveries, with most occurring during the early postpartum period. Treatment for distal SVT includes compression and analgesia. Proximal SVT, however, is associated with an increased risk of deep vein extension and may necessitate anticoagulation.7
Etiology of Venous Thromboembolism
VIRCHOW’S TRIAD
The elements of Virchow’s triad—venous stasis, vascular damage, and hypercoagulability—are all present during pregnancy and the postpartum period.
Venous Stasis Venous stasis begins in the first trimester and reaches a peak at 36 weeks’ gestation. Compression of the pelvic veins by the enlarged uterus as well as progesterone-induced venodilation contributes to this stasis. By 25 to 29 weeks’ gestation, venous flow velocity is reduced by approximately 50% in the legs and does not return to normal nonpregnancy flow velocity rates until around 6 weeks’ postpartum.
Vascular Damage Local damage to pelvic veins may occur during vaginal and cesarean delivery. The separation of the placenta results in vascular trauma.
Hypercoagulable State Reduction in the anticoagulant activity of protein S and enhanced resistance to the anticoagulant activity of protein C are noted. Increased levels of procoagulant factors, including factors V, VII, IX, X, and fibrinogen lead to enhanced thrombin production. In addition, thrombus dissolution is reduced through decreased fibrinolysis as a result of decreased activity of tissue plasminogen activator.1
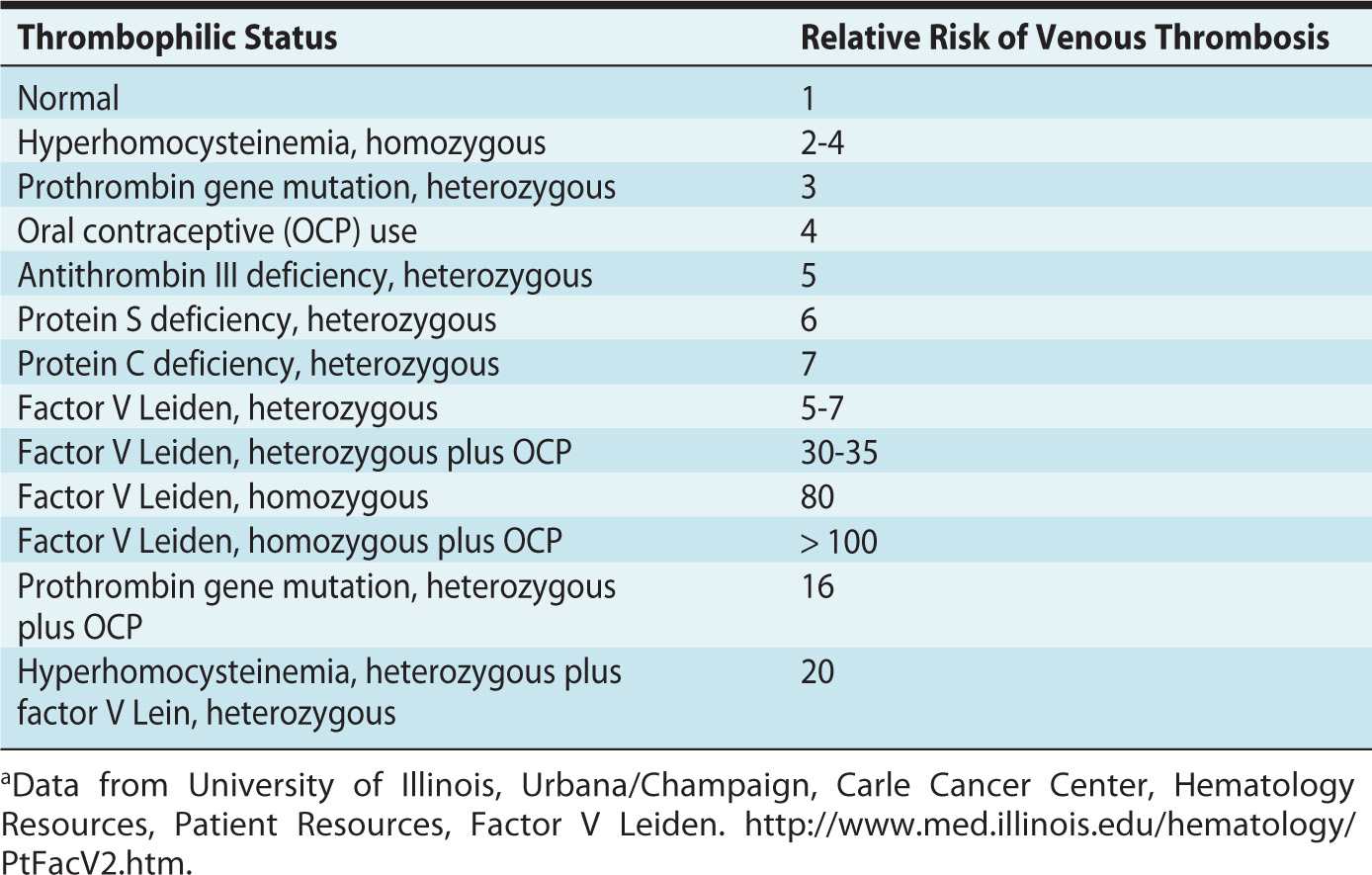
Thrombophilias are associated with a higher risk of venous thromboembolism during pregnancy. Approximately 50% of venous thromboembolic events in pregnancy are associated with inherited or acquired thrombophilia.2 The prevalence and relative risk of thrombosis depend on the type of thrombophilia (Table 19-1).
Table 19-1. Relative Risk of Venous Thrombosis Depending on Type of Thrombophiliaa
ADDITIONAL RISKS OF VENOUS THROMBOEMBOLISM
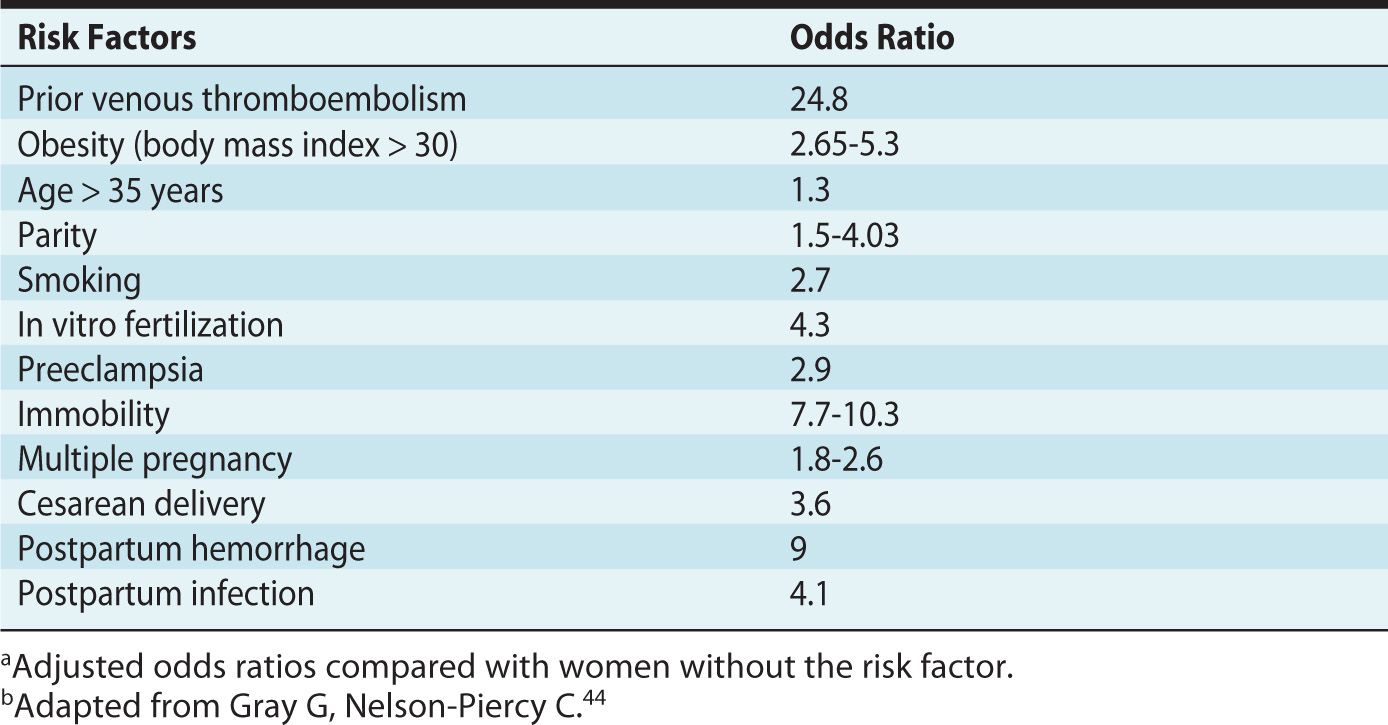
In addition to the elements of Virchow’s triad, identification of additional risk factors is useful for appropriate assessment on the need for thromboprophylaxis (Table 19-2).
Table 19-2. Risk Factors for Venous Thromboembolism in Pregnancya,b
Diagnosis of Venous Thromboembolism
CLINICAL
The most common signs of DVT are pain and tenderness of the leg, primarily in the left leg. Edema of thigh, erythema, palpable cord, and calf pain with passive dorsiflexion of thigh (Homans’ sign) may be present. Because an accurate clinical diagnosis of PE in pregnancy is difficult (due to the overlap of signs and symptoms between physiologic changes of pregnancy and development of PE), it is an important diagnosis to rule out in patients with related symptoms. Symptoms of PE include dyspnea (62%), pleuritic chest pain (55%), cough (24%) with or without hemoptysis, and diaphoresis (18%). Other clinical signs include tachycardia, tachypnea, hypoxemia, wheezing, decreased breath sounds, or fever. An accentuated second heart sound from right ventricular failure may be heard. With massive PE, defined as obstruction of more than 50% of the pulmonary circulation, hypotension, syncope, or cardiovascular collapse may be the presenting symptoms.2
DIAGNOSTIC TOOLS
Evaluation of PE in pregnancy attempts to balance timely and accurate diagnostic approach while minimizing fetal exposure to ionizing radiation. Pregnancy must not interfere with using the most appropriate diagnostic imaging studies for suspected PE because of the significant risk of morbidity and mortality for both mother and the fetus (Figure 19-2).2 The following studies may be useful:
• Compression ultrasound. This is the investigation of first choice in patients with suspected PE and positive signs of DVT, because it avoids the effect of radiation.
• Chest x-ray. If signs of DVT are absent, this is used to rule out other diagnoses for symptoms (ie, pneumothorax, pleurisy) that may preclude further diagnostic imaging.2
• Ventilation-perfusion scan. In the setting of a normal chest x-ray, a ventilation-perfusion scan is recommended. Technetium-labeled albumin injected intravenously is trapped in the pulmonary capillary bed, depicting the distribution of pulmonary blood flow. This perfusion scan is coupled to a ventilation scan to enhance specificity. The Prospective Investigation of Pulmonary Embolism Diagnosis was a large, prospective, multicenter trial that examined the diagnostic use of the ventilation-perfusion scan. They found that the combination of clinical assessment and the ventilation-perfusion scan improved diagnostic accuracy.8
• Computed tomography with pulmonary angiography. This is used in the setting of a nondiagnostic ventilation-perfusion scan.
• D-dimer. D-dimer levels have a high negative predictive value when used to rule out PE in the nonpregnant population.9 However, D-dimer levels increase gradually during pregnancy and drop in the immediate postpartum period to return to baseline by 6 weeks’ postpartum. Therefore, D-dimer levels are not recommended for exclusion of PE in the pregnant population.10
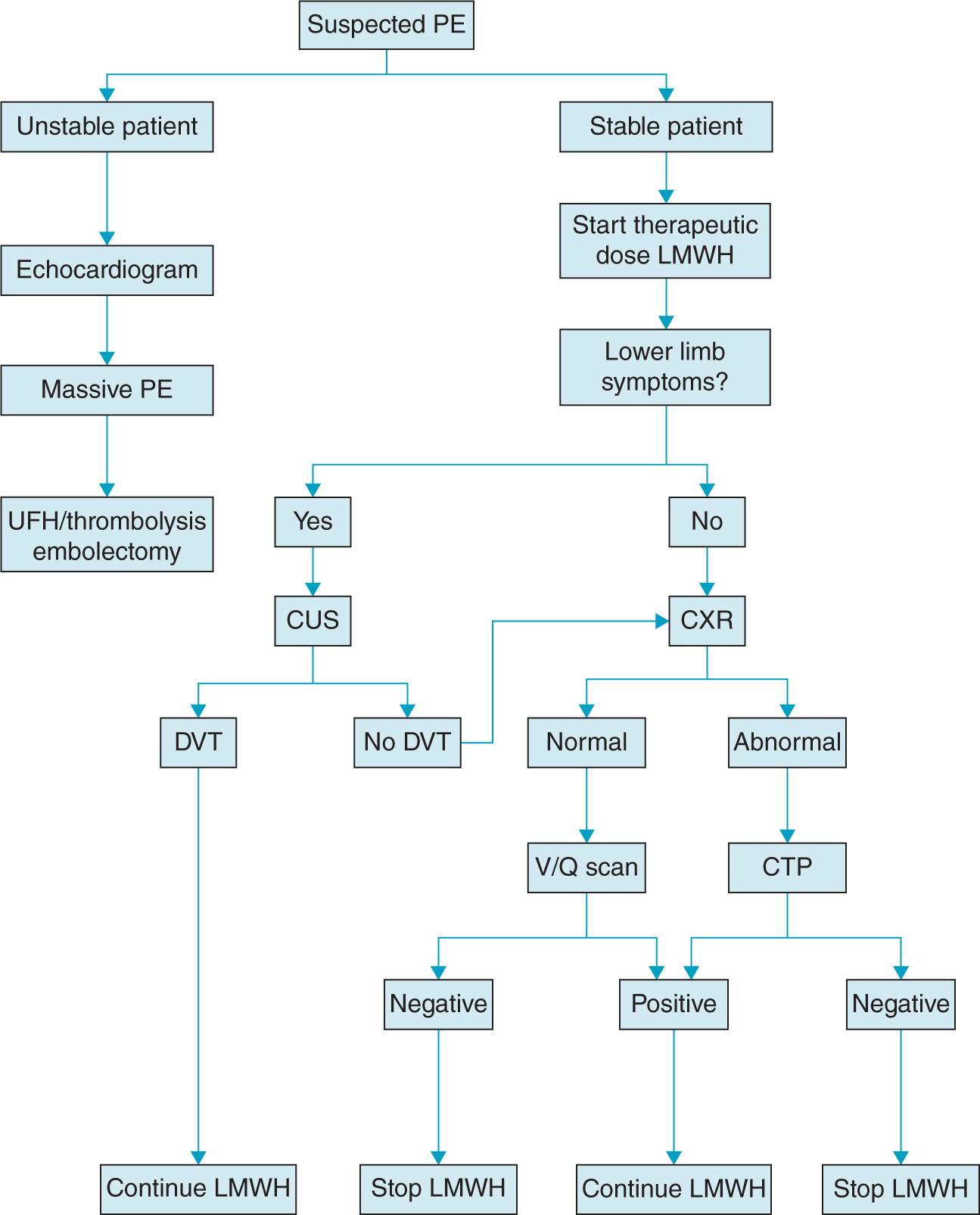
Figure 19-2. Proposed algorithm for diagnosis and treatment of pulmonary embolism in pregnancy. (From Gray G, Nelson-Piercy C,44 with permission.)
Thromboprophylaxis and Treatment
The prevalence and severity of this condition during pregnancy and the peripartum period necessitates special consideration not only for management of acute thrombotic events but for prophylaxis of those at increased risk of thrombotic events.4
THROMBOPROPHYLAXIS
Recurrence rates of VTE in pregnant patients without thromboprophylaxis are higher than those in nonpregnant patients (10.9% versus 3.7%).4 International societies such as the American College of Chest Physicians have recently published guidelines regarding use of antithrombotic agents in the management of VTE during pregnancy.8 Anticoagulation prophylaxis during pregnancy is recommended in patients with a previous episode of VTE or with history of thrombophilia.4 For most parturients, however, the benefits of anticoagulation do not outweigh the risk (2%) of bleeding complications from heparin or low-molecular-weight heparin.5
THERAPY
Anticoagulation is recommended for the treatment of established DVT or PE occurring during pregnancy and the postpartum period. Heparin, unfractionated or low molecular weight, is the anticoagulant of choice during pregnancy because it does not cross the placenta. Warfarin crosses the placenta and is considered teratogenic.
Deep Venous Thrombosis The current management approach for acute DVT during pregnancy is with unfractionated heparin (UFH) or low-molecular-weight heparin (LMWH). Advantages of UFH include a shorter half-life and ability to reverse with protamine sulfate. An advantage of LMWH is its more predictable dose-response, fewer bleeding episodes, lower risks of heparin-induced osteoporosis and heparin-induced thrombocytopenia.1 Dose adjustments are usually not necessary, and anti-Xa measurement does not need to be performed. Because UFH and LMWH are metabolized by both the kidney and liver, additional precaution is advised for those with renal or hepatic dysfunction. Current guidelines recommend LMWH over UFH for anticoagulation during pregnancy and postpartum.8
Pulmonary Embolism Acute treatment includes prompt therapeutic anticoagulation with intravenous heparin, support of maternal circulation, and provision of adequate oxygenation. Because two-thirds of PE-related morbidity occurs within 30 minutes of the acute event, if a patient has a strong clinical picture of PE, anticoagulation should be initiated before diagnostic studies are performed.2 Treatment is usually initiated with a loading dose of 110 to 120 U/kg of heparin intravenously, followed by a continuous infusion of 15 to 25 U/kg/h to maintain the activated partial thromboplastin time (PTT) at two times the normal value. If the patient requires delivery during the acute therapeutic treatment phase, full anticoagulation is discontinued and reversed with protamine once in active labor or in preparation for cesarean delivery.2
A vena cava filter is considered alongside anticoagulation if there is concern for continuing embolism or solely, if anticoagulation is contraindicated. For pregnant or postpartum patients, the filter should be placed in the suprarenal vena cava rather than standard infrarenal position as the left ovarian vein empties into the left renal vein.
Thrombolytic therapy is a relative contraindication in pregnancy. Its use should be avoided close to time of delivery and shortly postpartum because of the risk of hemorrhage. However, it may be appropriate and life saving for patients with massive PE and hemodynamic instability. Urokinase, streptokinase, and tissue plasminogen activator have been reported as successful thrombolytic agents in pregnant patients.11
Surgical embolectomy is used when thrombolytic therapy is contraindicated and PE is life threatening.
Anesthetic Management
Anticoagulated patients are at risk for bleeding complications associated with delivery. Management of anticoagulation near the time of delivery needs coordination between the provider and the patient to minimize risk and to plan for the anticipated regional anesthesia during labor and delivery. Ideally, those on prophylactic LMWH are switched to 5000 to 7500 units of UFH at least 1 to 2 weeks before anticipated labor or admission for induction or cesarean delivery. The patient is usually instructed to avoid her next scheduled injection of heparin when regular uterine contractions begin. On admission to labor, a clotting profile should be obtained.3
The American Society of Regional Anesthesia and Pain Medicine guidelines recommend12 that:
• Neuraxial anesthesia not be placed until 12 hours after the last dose of prophylactic and 24 hours after therapeutic LMWH dosing (enoxaparin 1 mg/kg every 12 hours or enoxaparin 1.5 mg/kg daily).
• Patients receiving heparin for more than 4 days have a platelet count assessed before neuraxial block and catheter removal to rule out heparin-induced thrombocytopenia.
• Indwelling neuraxial catheters be removed 2 to 4 hours after the last heparin dose and the patient’s coagulation status be assessed; heparin may be restarted 1 hour after catheter removal.
• The patient be monitored postoperatively to provide early detection of motor blockade, with considered use of minimal concentration of local anesthetics to enhance the early detection of a spinal hematoma.
• If on intravenous heparin, doses be stopped 6 hours before placement of a neuraxial block and a normal PTT be confirmed before proceeding with the block. If a cesarean section must be emergently carried out on patients without normalized PTT, the preferred method is general anesthesia.2
AMNIOTIC FLUID EMBOLISM
Amniotic fluid embolism (AFE) is a rare condition but responsible for a significantly high maternal mortality and morbidity.
Mechanism
EMBOLIC
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








