Electrolyte imbalances and drugs
Patients with electrolyte imbalances—such as hyperkalemia, hypokalemia, hypercalcemia, and hypocalcemia—frequently show distinctive rhythm changes on electrocardiograms (ECGs). Likewise, patients taking such drugs as digoxin (Lanoxin) may also exhibit characteristic ECG patterns that can provide early warnings of drug toxicity. Learning to recognize these variations early will help you identify and treat potentially dangerous conditions before they become serious.
Keep in mind, however, that the patient’s ECG is only part of the clinical picture. Additional information, such as the patient’s medical history, findings on physical examination, and additional diagnostic studies, are necessary to confirm an initial diagnosis based on ECG analysis.
Electrolyte Imbalances
Potassium and calcium ions play a major role in the heart’s electrical activity. Depolarization results from the exchange of these ions across the cell membrane. Changes in ion concentration can affect the heart’s electrical activity and, as a result, the patient’s ECG. This section discusses the effects of high and low potassium and calcium levels on an ECG.
Hyperkalemia
Potassium, the most plentiful intracellular cation (positively charged electrolyte), contributes to many important cellular functions. Most of the body’s potassium content is located in the cells. The intracellular fluid (ICF) concentration of potassium is 150 to 160 mEq/L; the extracellular
fluid (ECF) concentration, 3.5 to 5.0 mEq/L. Many symptoms associated with potassium imbalance result from changes in this ratio of ICF to ECF potassium concentration. Hyperkalemia is generally defined as serum potassium level greater than 5 mEq/L. It’s most commonly seen in people with renal insufficiency.
fluid (ECF) concentration, 3.5 to 5.0 mEq/L. Many symptoms associated with potassium imbalance result from changes in this ratio of ICF to ECF potassium concentration. Hyperkalemia is generally defined as serum potassium level greater than 5 mEq/L. It’s most commonly seen in people with renal insufficiency.
Causes
Increased intake of potassium—from excessive dietary intake, I.V. administration of penicillin G (Pfizerpen), potassium supplements, or banked whole blood—is one factor contributing to the development of hyperkalemia. Also, changes in cell membrane permeability or damage to cells from surgery, burns, massive crush injuries, cell hypoxia, acidosis, and insulin deficiency may cause potassium to shift from the intracellular space to the extracellular space. Increased serum levels of potassium may also stem from decreased renal excretion of potassium. This excretion may occur because of renal failure, decreased production and secretion of aldosterone, Addison’s disease, and the use of potassium-sparing diuretics.
Clinical significance
When extracellular potassium concentrations increase without a significant change in intracellular potassium concentrations, the cell becomes less negative, or partially depolarized, and the resting cell membrane potential decreases. Mild elevations in extracellular potassium result in cells that repolarize faster and are more irritable. More critical elevations in extracellular potassium result in an inability of cells to repolarize and respond to electrical stimuli. Cardiac standstill, or asystole, is the most serious consequence of severe hyperkalemia.
ECG characteristics
Rhythm: Atrial and ventricular rhythms are regular.
Rate: Atrial and ventricular rates are within normal limits.
P wave: In mild hyperkalemia, the amplitude is low; in moderate hyperkalemia, P waves are wide and flattened; in severe hyperkalemia, the P wave may be indiscernible.
PR interval: Normal or prolonged; not measurable if P wave can’t be detected.
QRS complex: Widened because ventricular depolarization takes longer.
ST segment: May be elevated in severe hyperkalemia.
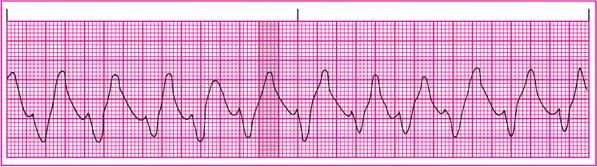
T wave: Tall, peaked; the classic and most striking feature of hyperkalemia.
QT interval: Shortened.
Other: Intraventricular conduction disturbances commonly occur. (See ECG effects of hyperkalemia.)
Signs and symptoms
Mild hyperkalemia may cause neuromuscular irritability, including restlessness, intestinal cramping, diarrhea, and tingling lips and fingers. Severe hyperkalemia may cause loss of muscle tone, muscle weakness, and paralysis.
Interventions
Treatment depends upon the severity of hyperkalemia and the patient’s signs and symptoms. The underlying cause must be identified and the extracellular potassium concentration brought back to normal. Drug therapy to normalize potassium levels includes calcium gluconate to decrease neuromuscular irritability, insulin and glucose to facilitate the entry of potassium into the cell, and sodium bicarbonate to correct metabolic acidosis.
Oral or rectal administration of cation exchange resins, such as sodium polystyrene sulfonate (Kayexalate), may be used to exchange sodium for potassium in the intestine. In the setting of renal failure or severe hyperkalemia, dialysis may be necessary to remove excess potassium. The patient’s serum potassium levels should be monitored closely until they return to normal, and arrhythmias should be identified and managed appropriately.
Hypokalemia
Hypokalemia, or potassium deficiency, occurs when the ECF concentration of potassium drops below 3.5 mEq/L, usually indicating a loss of total body potassium. The concentration of ECF potassium is so small that even minor changes in ECF potassium affect resting membrane potential.
Causes
Factors contributing to hypokalemia include increased loss of body potassium, increased entry of potassium into cells, and reduced potassium intake. Shifts in potassium from the extracellular space to the intracellular space may be caused by alkalosis, especially respiratory alkalosis. Intracellular uptake of potassium is also increased by catecholamines. Although rare, a dietary deficiency may contribute to hypokalemia in an elderly person. The condition is also seen in patients with alcoholism, hepatic disease, and anorexia nervosa. Potassium is also lost during diabetic ketoacidosis because of osmotic diuresis.
GI and renal disorders are the most common causes of potassium loss from body stores. GI losses of potassium are associated with laxative abuse, intestinal fistulae or drainage tubes, diarrhea, vomiting, and continuous nasogastric drainage.
Renal loss of potassium is related to increased secretion of potassium by the distal tubule. Diuretics, a low serum magnesium concentration, and excessive aldosterone secretion may cause urinary loss of potassium. In addition, several antibiotics, including gentamicin (Garamycin) and amphotericin B (Fungizone), are known to cause hypokalemia.
Clinical significance
When extracellular potassium levels decrease rapidly and intracellular potassium concentration doesn’t change, the resting membrane potential becomes more negative and the cell membrane becomes hyperpolarized. The cardiac effects of hypokalemia are related to these changes in membrane excitability. Ventricular repolarization is delayed because potassium contributes to the repolarization phase of the action potential. Hypokalemia can cause dangerous ventricular
arrhythmias and increases the risk of digoxin toxicity.
arrhythmias and increases the risk of digoxin toxicity.
ECG characteristics
Rhythm: Atrial and ventricular rhythms are regular.
Rate: Atrial and ventricular rates are within normal limits.
P wave: Usually normal size and configuration but may become peaked in severe hypokalemia.
PR interval: May be prolonged.
QRS complex: Within normal limits or possibly widened; prolonged in severe hypokalemia.
QT interval: Usually indiscernible as the T wave flattens.
ST segment: Depressed.
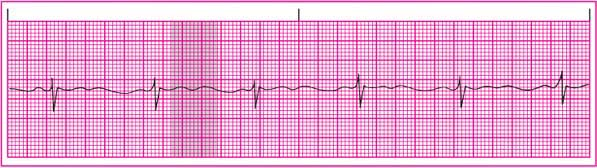
T wave: Amplitude is decreased. The T wave becomes flat as the potassium level drops. In severe hypokalemia, it flattens completely and may become inverted. The T wave may also fuse with an increasingly prominent U wave.
Other: Amplitude of the U wave is increased, becoming more prominent as hypokalemia worsens and fusing with the T wave. (See ECG effects of hypokalemia.)
Signs and symptoms
The most common symptoms of hypokalemia are caused by neuromuscular and cardiac effects, including smooth muscle atony, skeletal muscle weakness, and cardiac arrhythmias. Loss of smooth muscle tone results in constipation, intestinal distention, nausea, vomiting, anorexia, and paralytic ileus. Skeletal muscle weakness occurs first in the larger muscles of the arms and legs and eventually affects the diaphragm, causing respiratory arrest.
Cardiac effects of hypokalemia include arrhythmias, such as bradycardia, atrioventricular (AV) block, and paroxysmal atrial tachycardia. Delayed depolarization results in characteristic changes on the ECG.
Interventions
The underlying causes of hypokalemia should be identified and corrected. Acid-base imbalances should be corrected, potassium losses replaced, and further losses prevented. Encourage intake of foods and fluids rich in potassium. Oral or I.V. potassium supplements may be administered. The patient’s serum potassium levels should be monitored closely until they return to normal, and cardiac arrhythmias should be identified and managed appropriately.
Hypercalcemia
Most of the body’s calcium stores (99%) are located in bone. The remainder is found in the plasma and body cells. Approximately 50% of plasma calcium is bound to plasma proteins. About 40% is found in the ionized or free form.
Calcium plays an important role in myocardial contractility. Ionized calcium is more important than plasma-bound calcium in physiologic functions. Hypercalcemia is usually defined as a serum calcium concentration greater than 10.5 mg/dl.
Causes
The most common causes of hypercalcemia include excess vitamin D intake; bone metastasis and calcium resorption associated with cancers of the breast, prostate, and cervix; hyperparathyroidism; sarcoidosis; thyrotoxicosis; thiazide diuretic therapy; and many parathyroid hormone–producing tumors.
Clinical significance
In hypercalcemia, calcium is found inside cells in greater abundance than normal. The cell membrane becomes refractory to depolarization as a result of a more positive action potential, and a stronger stimulus is needed to cause a response in the cell membrane. This loss of cell membrane excitability causes many of the cardiac symptoms seen in patients with hypercalcemia.
Both ventricular depolarization and repolarization are accelerated. The patient may experience bradyarrhythmias and varying degrees of AV block.
ECG characteristics
Rhythm: Atrial and ventricular rhythms are regular.
Rate: Atrial and ventricular rates are within normal limits, but bradycardia can occur.
P wave: Normal size and configuration.
PR interval: May be prolonged.
QRS complex: Within normal limits, but may be prolonged.
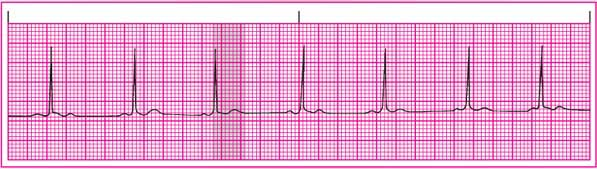
QT interval: Shortened.
ST segment: Shortened.
T wave: Normal size and configuration; may be depressed.
Other: None. (See ECG effects of hypercalcemia.)
Signs and symptoms
Common signs and symptoms of hypercalcemia include anorexia, nausea, constipation, lethargy, fatigue, polyuria, and weakness. Behavioral changes may also occur. Renal calculi may form as precipitates of calcium salts. Impaired renal function commonly occurs. A reciprocal decrease in serum phosphate levels commonly accompanies elevated levels of serum calcium.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree