Chapter 57
Drug Overdoses and Toxic Ingestions
Mechanisms of Injury
Direct Drug Effects
Nearly all drugs produce harmful effects if taken in excessive amounts. Systemic toxicity is due to selective effects of the toxin or a metabolite on specific targets, such as binding to specific receptors (therapeutic drugs), disruption of metabolic pathways (cyanide, salicylates, iron), cellular production of toxic metabolites (acetaminophen in the liver, methanol in the retina, ethylene glycol in the kidney), and enzymatic inhibition (Na+/K+-ATPase by digoxin; anticholinesterase by organophosphates). Some toxins produce effects by several mechanisms. For example, isoniazid causes both hepatotoxicity via a cytochrome P-450 pathway metabolite and neurotoxicity via the inhibition of pyridoxal 5′-phosphate. Pathologic effects may also occur at the site of exposure as a result of cytotoxic chemical reactions (e.g., caustic acid or alkali ingestions) that damage exposed tissue.
Complications
Aspiration occurs in poisoned patients as a complication of vomiting, orogastric lavage, endotracheal intubation, or loss of airway reflexes because of obtundation. Early assessment and definitive airway management are critical in diminishing the risk of aspiration. Acute lung injury may complicate recovery following life-threatening ingestions. Hyperthermia may occur for several reasons: increased motor activity that occurs with agitation or seizures, direct drug effects on the hypothalamus (sympathomimetics), or aspiration and pneumonia. Rhabdomyolysis (see Chapter 81) can occur in patients after prolonged periods of immobilization because of obtundation, protracted agitation or seizures, or cocaine or amphetamine use. Under these circumstances, aggressive hydration and maintenance of urine output are important. Acute renal failure (see Chapter 81) may occur directly, for example, from ethylene glycol direct toxic effects on the kidneys or secondarily, for example, from drug-induced hypotension. Acute hepatic failure (see Chapter 59) most commonly results from acetaminophen poisoning but may also occur because of the multiorgan effects of diffuse toxins such as mercury or iron.
Management
Therapeutic Approach
Gastrointestinal (GI) decontamination is no longer routinely recommended for most overdose patients but may have a limited role in some patients with serious toxicity admitted to the ICU. Orogastric lavage via a large-bore tube (Ewald tube) may be critical in patients ingesting large quantities of drugs not bound by activated charcoal, such as iron or lithium. It can be life saving in serious calcium channel antagonist overdoses by removing a clinically significant fraction of drug, decreasing toxicity. Orogastric lavage should only be considered in patients manifesting signs of toxicity following a potentially life-threatening ingestion, and only perform it after the judging the patient’s airway to be protected, often necessitating endotracheal intubation.
The regional poison control center should be consulted to obtain general management and toxin-specific therapeutic advice, as many common toxins have specific therapies or antidotes (Table 57.1).
TABLE 57.1
Antidotes and Adjuncts in the Therapy of Selected Poisonings
| Toxin | Antidote | Dosing for Adults and Comments |
| Acetaminophen | N-acetylcysteine | Orally 140 mg/kg × 1; followed by 70 mg/kg every 4 hours × 17 doses IV: 150 mg/kg IV over 60 minutes, followed by an infusion of 12.5 mg/kg/h over a 4-hour period, and finally an infusion of 6.25 mg/kg/h over a 16-hour period |
| Anticholinergic agents | Physostigmine | 1–2 mg IV over 5 minutes; use with caution for severe delirium (may cause seizures, bronchospasm, asystole, cholinergic crisis) |
| Beta-adrenergic antagonists | Glucagon | 2–5 mg IV; titrate repeat doses; may use infusion of 2–10 mg/h |
| Calcium channel blockers | Calcium gluconate | 1 g (10 mL of 10% solution) IV over 5 minutes with electrocardiographic monitoring; repeat as needed, check serum calcium after third dose |
| Insulin | Bolus dose of 0.1 U/kg followed by an infusion of 0.5 mg/kg/h; can be titrated up to a rate of 1 U/kg/h with a dextrose infusion to maintain euglycemia | |
| Cyclic antidepressants | Sodium bicarbonate | 1–2 mEq/kg IV; titrate to arterial pH of 7.5 or electrocardiographic alterations (see text) |
| Digoxin | Digoxin antibodies (Digibind) | Vials (number) = (digoxin level [ng/mL] × weight [kg])/100 or 10–20 vials for a life-threatening arrhythmia |
| Methanol Ethylene glycol | Fomepizole | Loading dose of 15 mg/kg IV over 30 minutes; subsequent 4 doses every 12 hours at 10 mg/kg; further dosing per poison center |
| Opioids | Naloxone | 0.05–0.4 mg IV, repeat as needed; infusion: two thirds of reversal dose/h, titrate to effect |
Common Toxic Ingestions
Acetaminophen
Acetaminophen is one of the most commonly ingested medications. Few patients become seriously ill from acetaminophen overdose because of early diagnosis and antidote treatment with N-acetylcysteine (NAC). Life-threatening hepatotoxicity, however, occurs in the few who present late after their ingestions or in whom clinicians fail to recognize acetaminophen when it is co-ingested with other drugs.
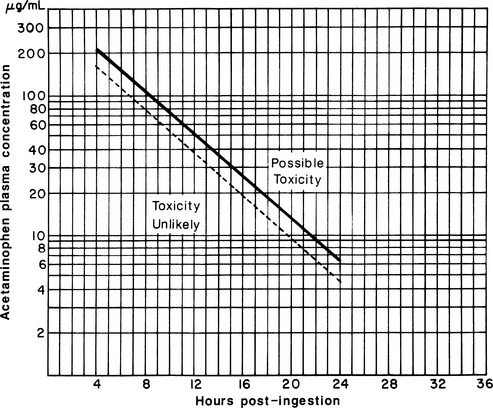
Patients with a history of acetaminophen ingestion should have a > 4-hour post ingestion acetaminophen level obtained and interpreted using the Rumack-Matthew nomogram (Figure 57.1). Nausea, vomiting, and sometimes right upper quadrant abdominal pain are associated with toxic hepatitis from ingestions a day or two earlier. Patients with jaundice or coagulopathy or those reporting a large acetaminophen ingestion 1 to 3 days previously should be presumed to have hepatotoxicity and should have treatment initiated immediately. When presentations are delayed more than 24 hours after ingestion, acetaminophen levels may be low or zero, but significant elevations in transaminases and prothrombin time reflect severe acetaminophen poisoning.
Therapeutic doses of acetaminophen are metabolized in the liver by glucuronidation (60%), sulfation (30%), or by the P-450 cytochrome oxidase system (4%). The last pathway results in a toxic intermediate, N-acetyl-p-benzoquinoneamine (NAPQI). NAPQI is then normally reduced by glutathione, which prevents toxicity. With increasing dose or overdose, more acetaminophen metabolism is shunted into the P-450 system, depleting glutathione. As a result, NAPQI accumulates and induces centrilobular necrosis of the liver. The antidote NAC replenishes the glutathione and prevents hepatic necrosis (Chapter 59).
Patients with toxic acetaminophen levels require a loading dose of NAC (140 mg/kg) and subsequent dosing every 4 hours (70 mg/kg) for an additional 17 doses over 72 hours. NAC can also be given parenterally with a loading dose of 150 mg/kg IV over 60 minutes then by continuous IV infusion over 20 hours (see Table 57.1). Although most effective within the first 8 hours after overdose, NAC therapy is effective up to 24 hours after overdose as well as in patients with fulminant hepatic failure secondary to acetaminophen. The current recommended dose and route of administration (orally versus IV) in these situations can be obtained via the local poison center. NAC should be continued until the acetaminophen level is zero and the liver function tests are trending down.
Alcohols
Laboratory testing should include finger-stick glucose, electrolytes, ethanol level with other alcohols, and serum osmolarity. Urine fluorescence with a Wood’s lamp can detect the presence of antifreeze (and presumably ethylene glycol) shortly after ingestion; however, this finding is not always present. An electrocardiogram (EKG) may show QT prolongation secondary to hypocalcemia from calcium oxalate precipitation in the kidneys.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree